37: Female Epispadias
This chapter will take approximately 15 minutes to read.
Introduction
Bladder exstrophy (BE) is a devastating congenital abnormality of the urinary tract in which infants are born with the urinary bladder extruded through their abdominal wall, a urethra that is open dorsally, and significant secondary abnormalities of the genitalia. It is widely regarded as the most surgically challenging and complex congenital disorder of the urinary tract.1 BE varies in degree of severity and exists on a continuum described as the bladder exstrophy-epispadias complex (BEEC).2 The incidence of the BEEC is ~1 in 10,000.2
Cloacal exstrophy (CE) is on the severe end of the BEEC spectrum. CE is a rare congenital disorder with an incidence of 1 in 200,000–400,000 live births.3,4 Common associated anomalies include omphalocele, bladder exstrophy, imperforate anus, and spinal dysraphism.4 Additional features include a midline plate of exstrophied cecum flanked by 2 bladder halves, a segment of ileum prolapsing from the cecal plate and a blind ending, rudimentary hindgut accompanied by an imperforate anus.4
On the opposite end of the spectrum lies epispadias. Epispadias is most commonly present as a component of BE and CE, but when epispadias occurs alone, in the absence of BE and CE it is regarded as the least severe variant of the BEEC. Epispadias is defined by an incomplete fusion of the dorsal urethra.5 This ranges from a mild glanular defect in a covered penis to the penopubic variety that extends proximally to the bladder neck and results in complete incontinence in males or females.5 Interestingly, the first described case of epispadias is attributed to the Byzantine emperor Heraclius (CE 610–641).6 Isolated male epispadias is a rare anomaly, with a reported incidence of 1 in 117,000 males.6 Isolated female epispadias is even more rare with an incidence of 1 in 484,000 female patients.6 In this chapter we will focus exclusively on female epispadias.
In 1928, Davis classified female epispadias into three categories based on severity. This classification system is still used today.7 In the lesser degree of epispadias the urethral orifice appears merely patulous. In intermediate epispadias the urethra is dorsally split along most of the urethra. In the most severe degree of epispadias the urethral cleft involves the entire length of the urethra and the sphincteric mechanism is rendered incompetent. Of note, the true prevalence of female epispadias may be higher than what is reported as in less severe cases the changes of the external genitalia may be minimal and if incontinence is not present patients go undiagnosed.8,9
Embryology
One of the leading theories of embryonic maldevelopment in exstrophy, proposed by Marshall and Muecke, is that the basic defect is an abnormal overdevelopment of the cloacal membrane during the fourth week of gestation, which prevents medial migration of the mesenchymal tissue and proper lower abdominal wall development. The timing of the rupture of this defective cloacal membrane is thought to determine the resulting variant.10 There is thought to be a failure of the cloacal membrane to be reinforced by ingrowth of mesoderm. The cloacal membrane is a bilaminar layer situated at the caudal end of the germinal disk that occupies the infraumbilical abdominal wall. Mesenchymal ingrowth between the ectodermal and endodermal layers of the cloacal membrane results in formation of the lower abdominal muscles and the pelvic bones. The cloacal membrane is subject to premature rupture, and if this results in a small infraumbilical defect, or the rupture occurs at a later stage in development then it is thought that epispadias will result, while in instances of larger defects or earlier rupture, BE or CE can result.10,11
Other plausible theories concerning the cause of the exstrophy-epispadias complex exist. Abnormal development of the genital hillocks caudal to the normal position, with fusion in the midline
below rather than above the cloacal membrane, has been embraced by other investigators.11,12 Another interesting hypothesis that remains controversial describes an abnormal caudal insertion of the body stalk, which results in a failure of interposition of the mesenchymal tissue in the midline.13 As a consequence of this failure, translocation of the cloaca into the depths of the abdominal cavity does not occur. A cloacal membrane that remains in a superficial infraumbilical position represents an unstable embryonic state with a strong tendency to disintegrate, which has been supported by the laboratory work of Thomalla et al.14,15 Another interesting theory is that maldevelopment of the bony pelvis, rather, is the inciting factor for the development of exstrophy. Beaudoin et al have suggested that lack of “rotation” of the pelvic ring primordium prevents structures attached to the pelvic ring from joining in the midline, allowing herniation of the bladder to occur.16
Epidemiology
Complete female epispadias is an extremely rare congenital defect, occurring in about 1/484,000 live births, much less frequently than BE, which is estimated to occur in 1/10,000 to 1/50,000.6 The male-to-female ratio of bladder exstrophy derived from multiple series is 2.3:1 and female epispadias is also less common than male epispadias which has a reported incidence of 1 in 117,000 males.17
The risk of recurrence of BE in a given family is approximately 1 in 100.18 Shapiro et al in a questionnaire identified the recurrence of BE and epispadias in only 9 of approximately 2,500 indexed cases.19 Shapiro et al determined that the risk of BE in the offspring of individuals with BE and epispadias is 1 in 70 live births, a 500-fold greater incidence than in the general population.17
Pathogenesis
Our current understanding of the pathogenesis of epispadias is incomplete. Due to a scarcity of quality research, experts are still unsure of the embryologic origins and genetic mechanisms that give rise to epispadias. However, strides have been made in recent years with respect to identifying candidate genes in the BEEC, which would cover the development of isolated epispadias as well. von Lowtow et al assessed 169 individuals with BEEC (recruited largely from Europe, geo-ethnic genetic origin not reported).20 They identified several individuals with pathogenic copy number variants, including one with a previously reported duplication in 22q11. This region is also associated with 22q11 Deletion Syndrome (DiGeorge Syndrome), and CAKUT.21 A subsequent genome-wide association study (GWAS) meta-analysis combining 568 BEEC patients and 3,241 controls of European origin identified an association with a locus containing the transcriptional enhancer ISL1 (p = 2.22 × 10−08).22,23,24,25,26 Further functional and model studies reinforced a possible causal role for ISL1 in BEEC. For example, developmental biology models were used to clarify the location of ISL1 activity in the forming urinary tract.26 Genetic lineage analysis of ISL1-expressing cells by a lineage tracer mouse model showed ISL1-expressing cells in the urinary tract of mouse embryos.26
Evaluation and Diagnosis
Even with modern ultrasound modalities, the prenatal diagnosis of BE is often difficult to delineate, and the prenatal diagnosis of isolated epispadias is nearly impossible.27 In 2012 Goyal et al. found that only 25% of cases of BEEC are diagnosed prenatally.27 Several groups have attempted to outline important criteria for the diagnosis of BE prenatally but there are no specific features for epispadias. They have found that for classic BE, the absence of a normal fluid-filled bladder on repeated examinations suggests the diagnosis, as does a mass of echogenic tissue on the lower abdominal wall.28,29 An umbilical cord insertion-to-genital tubercle length below the fifth percentile for gestational age also provides a quantitative measurement by which a BE diagnosis can be assessed.30 In a review of 25 prenatal ultrasound examinations with the subsequent birth of a newborn with BE, Gearhart et al made several observations:1 absence of bladder filling,2 a low-set umbilicus,3 widening pubis ramus,4 diminutive genitalia, and5 a lower abdominal mass that increased in size as the pregnancy progressed and as the intra-abdominal viscera increased in size.31 It is not hard to understand why the prenatal diagnosis of isolated epispadias is more difficult. Of those previously mentioned prenatal observations, only a low-set umbilicus, widened pubic ramus, and diminutive genitalia would potentially be present in the setting of isolated epispadias. All those findings would be less severe in the setting of isolated epispadias, and diminutive genitalia would not be applicable in the setting of isolated female epispadias.
As a result, the diagnosis of isolated female epispadias is generally made by physical exam postnatally. Changes to the external appearance of the genitalia may be minimal, particularly in cases of mild epispadias. Externally a genital defect marked by a bifid clitoris will be identified. The mons will be depressed in shape and coated by a smooth, glabrous area of skin. Beneath this area, there may be a moderate amount of subcutaneous tissue and fat, or the skin may be directly anterior and inferior to the surface of the symphysis pubis. The labia minora are usually poorly developed and terminate anteriorly at the corresponding half of the bifid clitoris, where there may be a rudiment of a preputial fold. The symphysis pubis is usually closed but may be separated by a narrow fibrous band. On separation of the labia, the urethral abnormality will be identified which can vary considerably depending on the degree of epispadias present. The urethral abnormality will range from a short distal defect to one that extends proximally to the bladder neck (Figure 1). The vagina and internal genitalia are usually normal. Because the external appearance changes may be minimal, some children are identified only because of persistent incontinence, and, in mild cases in which incontinence is not present, the diagnosis might never be made. That is the reason why the true prevalence of female epispadias may be higher than what is reported.8,9 Thus, isolated female epispadias always involves an anomaly of the urethra, but there can be coexisting abnormalities of the bladder neck that cannot be ignored when managing these patients. In more severe forms of female epispadias the bladder can be small and can be comparable to the bladders of patients with bladder exstrophy that has been closed, since there was no cycling of the bladder in utero. In fact, a third of all incontinent epispadias patients have a bladder capacity of less than 60 mL and the incidence of vesicoureteral reflux is 30% to 75%.32
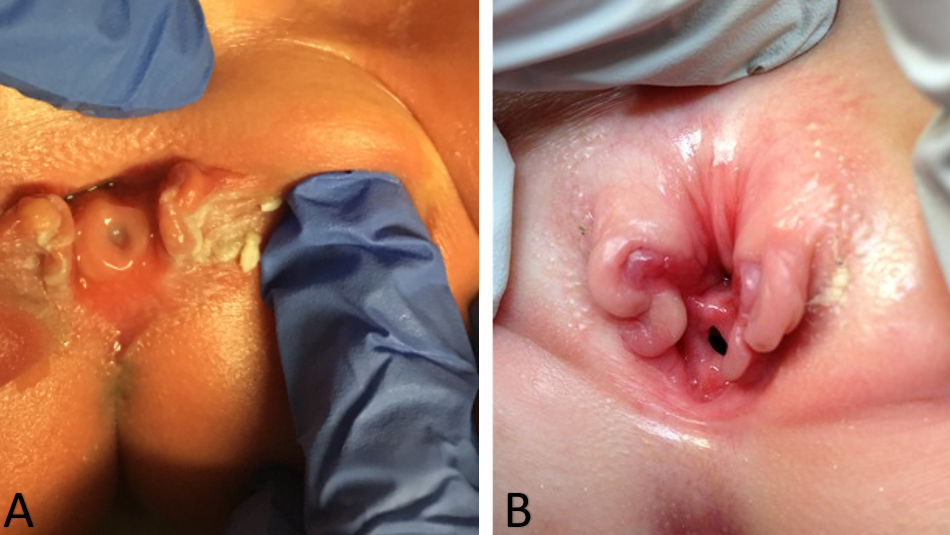
Figure 1 Severe cases of female epispadias. Penopubic epispadias in a newborn (A). Penopubic epispadias in a 4 year old (B).
Treatment and Outcomes
In the female epispadias patient the goals of treatment are achievement of urinary continence, preservation of the upper urinary tracts, and reconstruction of functional and cosmetically acceptable external genitalia. In patients with mild epispadias who are dry and have only minimal changes to their external genitalia surgical reconstruction may not be necessary. For more severe forms of female epispadias surgical reconstruction is required to obtain continence and correct abnormalities of the external genitalia.
Historically, there were many operations reported to attempt to achieve continence in the epispadias group, but the results were disappointing. These procedures included transvaginal plication of the urethra and bladder neck, muscle transplantations, urethra twisting, cauterization of the urethra, bladder flap, and Marshall-Marchetti vesicourethral suspension.33,34,35,36 These procedures sought to increase urethral resistance, but did not correct incontinence as they did not attempt to normalize the anatomy of the urethra and bladder neck.
As previously mentioned, patients with distal epispadias without incontinence may not require any surgical intervention. In cases of proximal female epispadias we will often perform epispadias repair, and bladder neck reconstruction, with or without osteotomies at the same time (Figure 2). This is similar to the complete primary repair of exstrophy that we perform in BE cases and is generally performed between 3–6 months of life. We have found that the added resistance gained from epispadias repair and bladder neck reconstruction allows for bladder cycling and can often result in an increase in bladder capacity. These findings have been corroborated by other authors who have shown that primary closure of the epispadiac urethra in children with closed exstrophy was found to increase bladder capacity without causing hydronephrosis. This approach has been applied to male and female patients with epispadias.37,38,39
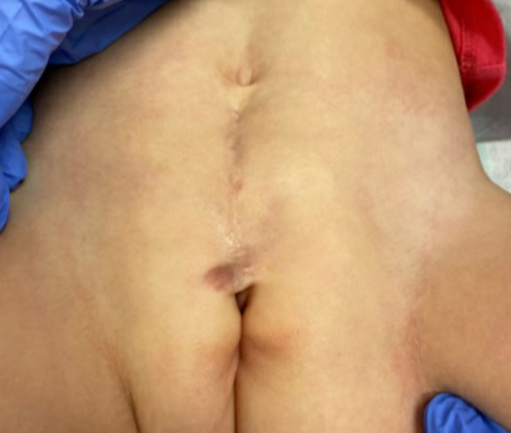
Figure 2 Post-operative image of a patient with severe female epispadias status post epispadias repair and bladder neck reconstruction.
Continence rates in females with isolated epispadias have been found to be between 67-87.5%.6,32,40 The mean interval after reconstruction until continence has been quoted to be between 18 and 30 months.6,41 There have also been rare reports of patients have delayed complete continence several years after reconstruction.32 The extended delay in achieving continence has been postulated to be secondary to increased pelvic muscular development over time and especially after the hormonal changes during puberty.32
Complications
The surgical management of epispadias is notoriously challenging and, as a result, complications are not uncommon. Potential complications following the repair of the epispadiac urethra include urethrocutaneous fistula, meatal stenosis and urethral strictures. For patients who require a bladder neck procedure the importance of close follow up cannot be overstated. When the outlet resistance of the bladder is tightened, patients are at risk of developing a high-pressure bladder due to the bladder contracting to fight against the high resistance, that places their upper tracts as risk. It is thus critical for surgeons to follow patients very closely following reconstruction in order to identify new onset hydronephrosis or other signs of a high-pressure system early. This is especially important in patients who had small, non-compliant bladders pre-operatively, although this is very rare in epispadias. Additionally, following a bladder neck procedure, patients may still suffer from incontinence and may require additional procedures to achieve continence.
In patients with severe forms of proximal epispadias bilateral iliac osteotomies may be required at the time of urologic reconstruction to take tension off of the closure by decreasing the pubic diastasis. Risks associated with bilateral iliac osteotomies include blood loss decreased perfusion to the lower extremities, and in very severe cases decreased perfusion to the clitoris. The decreased perfusion to the clitoris may occur when the pubic bones are brought together too tightly following osteotomies. Vesicocutaneous fistulas are also possible in patients who require bladder neck reconstruction. Interestingly, when Suson et al compared 22 girls with complete epispadias with 23 girls with classic BE they did not find a difference in number of surgical procedures, development of continence with or without the need for bladder neck reconstruction, or eventual need for continent urinary diversion.5
Suggested Follow Up
Patients with distal epispadias who void normally and have healthy upper tracts can often simply be monitored and potentially discharged from care after being followed for a period of time. Alternatively, patients with more severe forms of epispadias who undergo reconstruction should be followed throughout childhood and into adulthood. It is critical to transfer their care in adulthood to a transitional urologist who has experience in treating patients with complex congenital urologic anomalies.
As mentioned previously, patients who undergo epispadias repair and bladder neck reconstruction require very close follow up post operatively. Studies have shown that patients who undergo isolated bladder neck procedures without concomitant augmentation are at risk of developing elevated bladder pressures and subsequent upper tract deterioration. This has been shown to occur even when extensive pre-operative work up is performed to ensure safe pre-operative bladder pressures and compliance. Despite preoperative selection, there are significant changes in bladder dynamics and 40% of neurogenic bladder patients required subsequent augmentation.42 Thus, annual follow up with renal bladder ultrasounds is absolutely critical in these patients.
Conclusions
Isolated female epispadias is an exceedingly rare condition and is regarded as the least severe variant of the BEEC. It varies from a mild distal defect that does not affect continence, to a complete phenotype which extends proximally to the bladder neck and results in complete incontinence.5 Similar to BE, proximal epispadias is widely regarded as one the most surgically challenging and complex congenital disorder of the urinary tract.1 Epispadias repair, bladder neck reconstruction, and even osteotomies may be required in the most severe forms. Many epispadias patients will require lifelong follow up with a urologist who has experience in caring for patients with complex urologic anomalies.
Key Points
- Epispadias is the least severe variant of the BEEC and is defined by an incomplete fusion of the dorsal urethra.
- Complete female epispadias is an extremely rare congenital defect, occurring in about 1/484,000 live births, much less frequently than BE, which is estimated to occur in 1/10,000 to 1/50,000.
- The diagnosis of isolated female epispadias is generally made by physical exam postnatally as diagnosis via prenatal imaging is not reliable.
- The urethral abnormality can range from a short distal defect to one that extends proximally to the bladder neck
- In the female epispadias patient the goals of treatment are achievement of urinary continence, preservation of the upper urinary tracts, and reconstruction of functional and cosmetically acceptable external genitalia.
- In patients with mild epispadias who are dry and have only minimal changes to their external genitalia surgical reconstruction may not be necessary.
- For more severe forms of female epispadias surgical reconstruction is required to obtain continence and correct abnormalities of the external genitalia.
- Continence rates in females with isolated epispadias have been found to be between 67-87.5%.
References
- Weiss DA, Shukla AR, Borer JG, Sack BS, Kryger JV, Roth EB, et al.. Evaluation of outcomes following complete primary repair of bladder exstrophy at three individual sites prior to the establishment of a multi-institutional collaborative model. J Pediatr Urol 2020; 16 (4): 435.e1–435.e6. DOI: 10.1016/j.jpurol.2020.05.153.
- Reutter H, Keppler-Noreuil K, E. Keegan C, Thiele H, Yamada G, Ludwig M. Genetics of Bladder-Exstrophy-Epispadias Complex (BEEC): Systematic Elucidation of Mendelian and Multifactorial Phenotypes. Curr Genomics 2005; 17 (1): 4–13. DOI: 10.2174/1389202916666151014221806.
- Phillips TM. Spectrum of cloacal exstrophy. Semin Pediatr Surg 2011; 20 (2): 113–118. DOI: 10.1053/j.sempedsurg.2010.12.007.
- Woo LL, Thomas JC, Brock JW. Cloacal exstrophy: A comprehensive review of an uncommon problem. J Pediatr Urol 2010; 6 (2): 102–111. DOI: 10.1016/j.jpurol.2009.09.011.
- Suson KD, Preece J, Baradaran N, Di Carlo HN, Gearhart JP. The Fate of the Complete Female Epispadias and Exstrophy Bladder–Is There a Difference? J Urol 2013; 190 (4s): 1583–1589. DOI: 10.1016/j.juro.2013.01.093.
- Gearhart JP. Exstrophy-Epispadias Complex in Campbell-Walsh-Wein Urology 2021. Elsevier; .
- Davis DM. Epispadias in Females and Its Surgical Treatment. J Urol 1928; 20 (6): 673–678. DOI: 10.1016/s0022-5347(17)73196-5.
- Yeni E, Unal D, Verit A, Karatas OF. An adult female epispadias without exstrophy was presented with urinary incontinence: a case report. Int Urogynecol J 2004; 15 (3): 212–213. DOI: 10.1007/s00192-004-1131-2.
- Krishna Shetty MV, Sen TK, Bhaskaran VA. Female epispadias. Afr J Paediatr Surg 2011; 8 (2): 215. DOI: 10.4103/0189-6725.86066.
- Muecke EC. The Role of the Cloacae Membrane in Exstrophy: The First Successful Experimental Study. J Urol 1964; 92 (6): 659–668. DOI: 10.1016/s0022-5347(17)64028-x.
- Ambrose SS, O’Brien DP. Surgical Embryology of the Exstrophy-Epispadias Complex. Surg Clin North Am 1974; 54 (6): 1379–1390. DOI: 10.1016/s0039-6109(16)40493-7.
- Patten BM, Barry A. The genesis of exstrophy of the bladder and epispadias. Am J Anat 1952; 90 (1): 35–57. DOI: 10.1002/aja.1000900103.
- Mildenberger H, Kluth D, Dziuba M. Embryology of bladder exstrophy. J Pediatr Surg 1988; 23 (2): 166–170. DOI: 10.1016/s0022-3468(88)80150-7.
- Johnston JH, Kogan SJ. The exstrophic anomalies and their surgical reconstruction. Curr Probl Surg 1974; 11 (8): 1–39. DOI: 10.1016/s0011-3840(74)80011-0.
- Thomalla JV, Rudolph RA, Rink RC, Mitchell ME. Induction of Cloacal Exstrophy in the Chick Embryo Using the CO 2 Laser. J Urol 1985; 134 (5): 991–995. DOI: 10.1016/s0022-5347(17)47573-2.
- Beaudoin S, Simon L, Bargy F. Anatomical basis of a common embryological origin for epispadias and bladder or cloacal exstrophies. Surg Radiol Anat 1997; 19 (1): 11–16. DOI: 10.1007/bf01627728.
- Shapiro E, Lepor H, Jeffs RD. The Inheritance of the Exstrophy-Epispadias Complex. J Urol 1984; 132 (2): 308–310. DOI: 10.1016/s0022-5347(17)49605-4.
- Ives E, Coffey R, Carter CO. A family study of bladder exstrophy. J Med Genet 1980; 17 (2): 139–141. DOI: 10.1136/jmg.17.2.139.
- Shapiro E, Jeffs RD, Gearhart JP, Lepor H. Muscarinic Cholinergic Receptors in Bladder Exstrophy: Insights Into Surgical Management. J Urol 1985; 134 (2): 308–310. DOI: 10.1016/s0022-5347(17)47139-4.
- Reutter H, Boyadjiev SA, Gambhir L, Ebert A-K, Rösch WH, Stein R, et al.. Phenotype Severity in the Bladder Exstrophy-Epispadias Complex: Analysis of Genetic and Nongenetic Contributing Factors in 441 Families from North America and Europe. J Pediatr 2011; 159 (5): 825–831.e1. DOI: 10.1016/j.jpeds.2011.04.042.
- WOOD HADLEYM, TROCK BRUCEJ, GEARHART JOHNP. In Vitro Fertilization and the Cloacal-Bladder Exstrophy-Epispadias Complex: Is there an Association? J Urol 2003; 169 (4): 1512–1515. DOI: 10.1097/01.ju.0000054984.76384.66.
- Lowtzow C von, Hofmann A, Zhang R, Marsch F, Ebert A-K, Rösch W, et al.. CNV analysis in 169 patients with bladder exstrophy-epispadias complex. BMC Med Genet 2016; 17 (1): 35. DOI: 10.1186/s12881-016-0299-x.
- Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, et al.. Copy-Number Disorders Are a Common Cause of Congenital Kidney Malformations. Am J Hum Genet 2012; 91 (6): 987–997. DOI: 10.1016/j.ajhg.2012.10.007.
- Arkani S, Cao J, Lundin J, Nilsson D, Källman T, Barker G, et al.. Evaluation of the ISL1 gene in the pathogenesis of bladder exstrophy in a Swedish cohort. Hum Genome Var 2018; 5 (1): 18009. DOI: 10.1038/hgv.2018.9.
- Draaken M, Knapp M, Pennimpede T, Schmidt JM, Ebert A-K, Rösch W, et al.. Genome-wide Association Study and Meta-Analysis Identify ISL1 as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy. PLoS Genet 2015; 11 (3): e1005024. DOI: 10.1371/journal.pgen.1005024.
- Reutter H, Draaken M, Pennimpede T, Wittler L, Brockschmidt FF, Ebert A-K, et al.. Genome-wide association study and mouse expression data identify a highly conserved 32 kb intergenic region between WNT3 and WNT9b as possible susceptibility locus for isolated classic exstrophy of the bladder. Hum Mol Genet 2014; 23 (20): 5536–5544. DOI: 10.1093/hmg/ddu259.
- Reutter H, Hoischen A, Ludwig M, Stein R, Radlwimmer B, Engels H, et al.. Genome-wide analysis for micro-aberrations in familial exstrophy of the bladder using array-based comparative genomic hybridization. BJU Int 2007; 100 (3): 646–650. DOI: 10.1111/j.1464-410x.2007.07086.x.
- Zhang R, Knapp M, Suzuki K, Kajioka D, Schmidt JM, Winkler J, et al.. ISL1 is a major susceptibility gene for classic bladder exstrophy and a regulator of urinary tract development. Sci Rep 2017; 7 (1): 42170. DOI: 10.1038/srep42170.
- Goyal A, Fishwick J, Hurrell R, Cervellione RM, Dickson AP. Antenatal diagnosis of bladder/cloacal exstrophy: Challenges and possible solutions. J Pediatr Urol 2012; 8 (2): 140–144. DOI: 10.1016/j.jpurol.2011.05.003.
- Mirk P, Calisti A, Fileni A. Prenatal sonographic diagnosis of bladder extrophy. J Ultrasound Med 1986; 5 (5): 291–293. DOI: 10.7863/jum.1986.5.5.291.
- VERCO PW, KHOR BH, BARBARY J, ENTHOVEN C. Ectopia Vesicae in Utero. Australas Radiol 1986; 30 (2): 117–120. DOI: 10.1111/j.1440-1673.1986.tb02400.x.
- Fishel-Bartal M, Perlman S, Messing B, Bardin R, Kivilevitch Z, Achiron R, et al.. Early Diagnosis of Bladder Exstrophy: Quantitative Assessment of a Low-Inserted Umbilical Cord. J Ultrasound Med 2017; 36 (9): 1801–1805. DOI: 10.1002/jum.14212.
- GEARHART J, BENCHAIM J, JEFFS R, SANDERS R. Criteria for the prenatal diagnosis of classic bladder exstrophy. Obstet Gynecol 1995; 85 (6): 961–964. DOI: 10.1016/0029-7844(95)00069-4.
- Kelalis PP, Kramer SA. Surgical Correction of Female Epispadias. Eur Urol 1982; 8 (6): 321–324. DOI: 10.1159/000473547.
- Jonuzi A, Popović N, Zvizdić Z, Milišić E, Karavdić K, Dewan P. Female Epispadias Presenting as Urinary Incontinence. APSP J Case Rep 2017; 8 (2): 10. DOI: 10.21699/ajcr.v8i2.548.
- Gross RE, Cresson SL. Exstrophy Of Bladder. J Am Med Assoc 1952; 149 (18): 1640. DOI: 10.1001/jama.1952.02930350028008.
- MARSHALL VICTORFRAY, MARCHETTI ANDREWA, KRANTZ KERMITE. The Correction of Stress Incontinence by Simple Vesicourethral Suspension. J Urol 1949; 88 (4): 1326–1331. DOI: 10.1097/00005392-200210010-00005.
- &Na;. Epispadias and Incontinence. Plast Reconstr Surg 1983; 37 (5): 468. DOI: 10.1097/00006534-196605000-00023.
- Ben-Chaim J, Peppas DS, Jeffs RD, Gearhart JP. Complete Male Epispadias: Genital Reconstruction and Achieving Continence. J Urol 1995; 153 (5): 1665–1667. DOI: 10.1016/s0022-5347(01)67499-8.
- Gearhart JP, Jeffs RD. Bladder Exstrophy: Increase in Capacity Following Epispadias Repair. J Urol 1989; 142 (2 Part 2): 525–526. DOI: 10.1016/s0022-5347(17)38804-3.
- Peters CA, Gearhart JP, Jeffs RD. Epispadias and Incontinence: The Challenge of the Small Bladder. J Urol 1988; 140 (5 Part 2): 1199–1201. DOI: 10.1016/s0022-5347(17)42001-5.
- Hanna MK, Williams DI. Genital Function In Males With Vesical Exstrophy And Epispadias. Br J Urol 1972; 44 (2): 169–174. DOI: 10.1111/j.1464-410x.1972.tb10062.x.
- Klauber GT, Williams DI. Epispadias with incontinence. Plast Reconstr Surg 1974; 54 (4): 504. DOI: 10.1097/00006534-197410000-00053.
- Weiss D. Faculty Opinions recommendation of Clinical outcomes after increasing bladder outlet resistance without augmentation cystoplasty in neurogenic bladder. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2021; 17 (2): 235 1–235 7. DOI: 10.3410/f.739262243.793583985.
Last updated: 2025-09-25 12:10