33: 尿道下裂再手术
阅读本章大约需要 5 分钟。
引言
继发性尿道下裂一词专指在多次矫治性尿道下裂修复术后仍存在解剖学和功能性并发症的患者。这些并发症包括尿道狭窄、尿道皮肤瘘、龟头裂开、尿道裂开、持续性阴茎下弯以及龟头畸形。尿道下裂由三个主要特征所定义:腹侧位的尿道口、不同程度的阴茎下弯,以及由于背侧包皮过多而呈现的“兜帽样”包皮外观;上述各组成部分的严重程度范围很广。因此,对于在初次修复术后前来接受翻修手术的患者,识别导致失败的各个组成部分及其组合,对于在翻修手术中最大化疗效至关重要。此外,由于组织血供减少,瘘的复发和狭窄的发生率也更高。
另一个需要牢记的关键方面是,需进行二次修复的患者群体往往年龄更大,研究强调其心理共病和神经发育障碍的显著发生率。1,2 大多数尿道下裂‘残废者’平均经历了三次以上失败的尿道成形术尝试,而且失败往往在最初达到令人满意的结果后的许多年才出现。3 Anthony Mundy 在其社论综述中描述了在管理这些患者队列时这一挑战的巨大程度。他强调,关于尿道下裂修复失败的优质期刊论文几乎不可能写出来。4 在认识到原发性尿道下裂修复后的并发症时,不能采取‘一刀切’的做法,而需要个体化量身定制的策略。本章概述发生率和易感因素,并提出一个针对需要再次进行尿道下裂修复手术的儿童的总体管理算法。此外,还将介绍本机构优选采用的外科技术。本章还将回顾针对每一种常见的单项并发症的替代策略,这些并发症随后会导致需要进行再次手术。
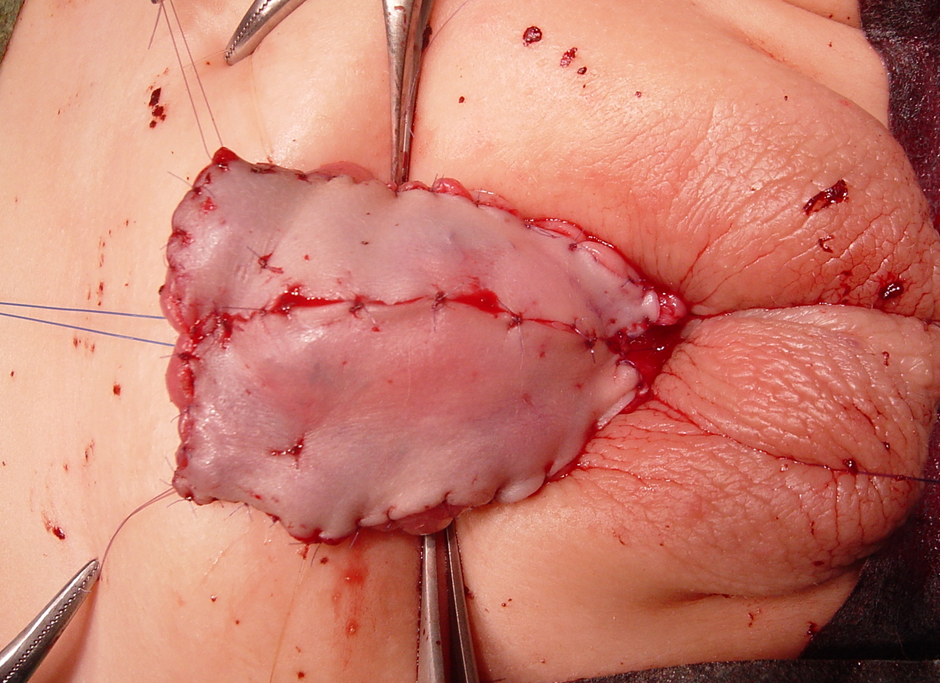
图 1 在 1st 期尿道下裂修复术中采用绗缝缝合固定的包皮皮片移植。请注意,龟头已被劈开并充分敞开。
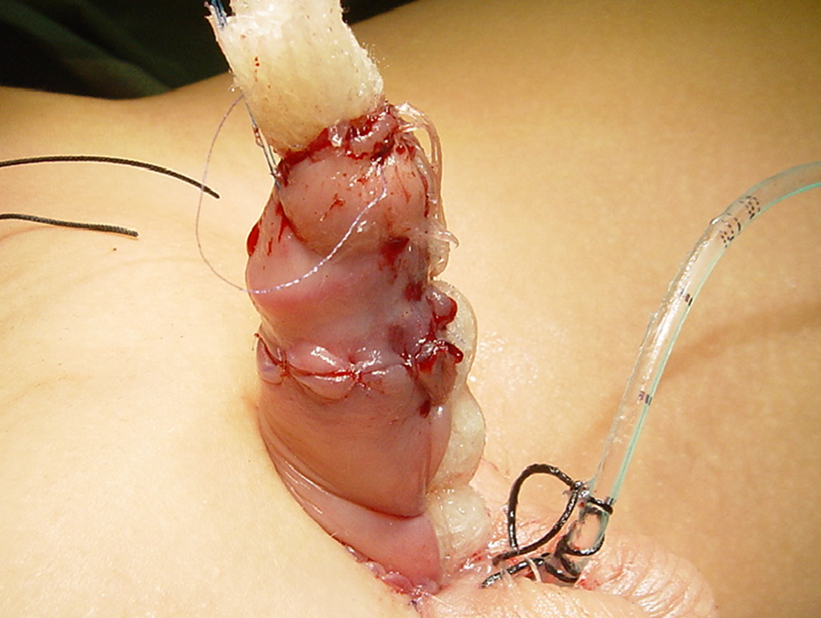
图 2 卷起的 jelonet 被固定,用于提供湿润并使皮肤移植物固定不动。
发病率
翻修手术主要受两大关键因素影响:机构的病例量和手术医师的技术经验。因此,各中心的发生率差异很大。Barbagli 等 在对单一青少年队列的回顾性分析中报告,50%的阴茎部尿道狭窄患者有尿道下裂修复失败的病史。在该队列中,16.4%曾因原发性远端型尿道下裂接受手术治疗,且多数人为修补原发缺陷而接受过不止一次手术。在人口学特征方面,21.2%的患者年龄为1至16岁,38.4%为17至20岁。5 这些发现凸显了长期随访的必要性,并强调了实现医疗照护有效衔接的需求。
危险因素
与初次修复者相比,接受第二次尿道下裂修复术的患者,其尿道成形术并发症发生率增加两倍。若再次手术达三次或以上,这些风险可增至40%。6 尿道下裂修复失败的病因病理是多因素的。术后感染、尿液外渗、残余瘢痕组织、血肿以及欠佳的手术技术,都会导致移植物的组织愈合受损与缺血。对于再次修复的尿道下裂,腹侧瘢痕化组织与周围可用组织的缺乏叠加,易致不良结局。7 理论上,青春期起始阶段,重建的新尿道的生长与周围海绵体组织的生长突增不匹配,可能导致管腔短而狭窄,表现为尿道狭窄。在儿科人群中,公认修复时年龄越大并发症风险越高。8 我们认为,二次修复应由经验丰富的尿道下裂外科医师在高手术量中心实施。有证据显示,手术量与患者结局呈正相关,尤其在这类患者中。9
诊断
对患儿的临床评估与主要表现为尿道下裂的患儿并无显著差异。如果患儿来自其他医疗中心,获取其既往手术的操作细节至关重要。在考虑进行修复时,应记录尿道外口位置、龟头体积、阴茎长度、复发性阴茎下弯(如有),以及尿道板的深度和宽度。同样关键的还有瘢痕组织的范围,以及在采取两期手术方案时可用于供移植物取材的供区部位的可用性。必须进行全面的体格检查并详尽记录,额外的影像学评估很少需要。患儿的个体特征以及术者的技术偏好与经验将影响修复方式的选择。最后,应将再次修复术的潜在风险与获益与父母共同讨论,实行共同决策。
围手术期计划
开展尿道下裂再修复手术在技术上具有挑战性,且与更高的并发症发生率相关。应明确告知,功能性结局始终优先于美容外观。“正常”的尿道口位置普遍被认为是龟头的远端顶端。然而,有报道指出,在未治疗的尿道下裂男性中,尽管尿道口位置差异显著,成年期的性功能和排尿功能仍可保持。10 除尿道口位置之外,一项调查发现,未治疗的“较轻”或远端型尿道下裂男性报告有性生活困难,主要与阴茎弯曲有关,但与正常男性相比并无显著的排尿问题。10 结合该调查结果并应用于二次修复的情境,阴茎弯曲(Chordee)矫正应作为首要考虑。如考虑采取两期手术方案,建议两次手术之间至少间隔6个月,以便伤口充分愈合。文献已报道了一些用于促进尿道下裂再修复组织愈合的辅助措施,尤其是硝酸甘油注射和高压氧治疗,以增强组织血供并促进创面愈合。11 然而,这些支持性治疗尚未得到广泛认可。在龟头较小、阴茎较短的儿童中,关于术前雄激素刺激的使用,业内仍在持续争论。有观点主张在重度尿道下裂中使用它,以增加阴茎长度、龟头周径、阴茎血供以及组织的韧性,这些在任何再修复中都至关重要。然而,也有相互矛盾的证据提示,在近端型尿道下裂中,术前激素刺激与并发症发生率增加相关,尽管在统计学上并无显著性差异。12
抗生素
我们主张在复修尿道下裂手术中给予抗生素覆盖,以尽量降低手术部位感染和泌尿道感染的风险。应在术前进行尿培养,如有菌尿,应依据培养及药敏结果进行治疗。尽管有研究发现,无论是否术前使用预防性抗生素,手术部位和泌尿道感染的发生率均无显著差异,13,14 我们首选静脉使用co-amoxiclav(阿莫西林/克拉维酸 30 mg/kg)作为我们的首选抗生素。随后在术后改为口服并持续一周,直至导尿管和敷料移除。我们的做法大体与一项研究一致:当留置尿道导管时,91%的小儿泌尿外科医师会开具术后抗生素。15 尽管尚有争议,最近一项评估尿道下裂修复术后预防性抗生素效果的荟萃分析发现,其在预防感染和伤口愈合并发症方面的效用有限,尽管许多研究存在较高的偏倚风险。16,17 为支持患者康复,提供充分镇痛至关重要,因为他们通常需要更广泛的组织分离和更复杂的重建。骶管阻滞是围手术期疼痛控制最常见的选择。或者,也可采用周围神经阻滞(阴茎背神经阻滞和阴部神经阻滞)。关于镇痛方式的选择及其与尿道下裂修复术后结局之间关系的证据仍不一致。18,19
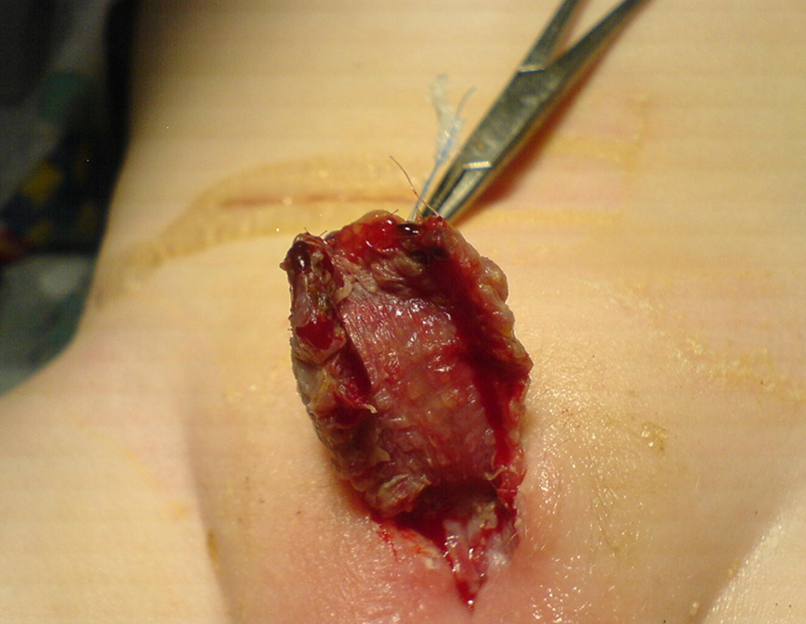
图 3 术后一周皮肤移植的外观。家庭照护者需要继续用凡士林或可可脂软膏按摩移植物,以优化愈合并预防移植物挛缩。
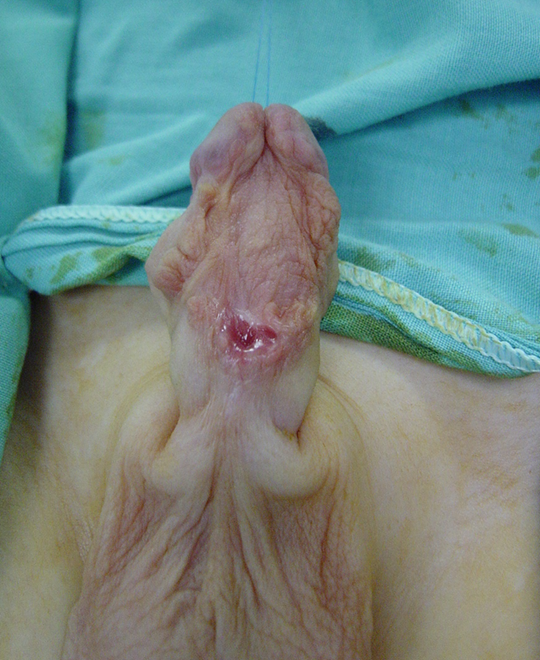
图 4 第1期修复术后6个月皮肤移植的外观。愈合良好,无移植皮片挛缩的证据。已可进行第2期修复。
手术技术
在尿道下裂再修复中,已报道多种挽救性技术,包括切开板管状化尿道成形术(TIP)、Mathieu修复术、贴敷式管状化岛状皮瓣(OIF)以及游离移植,可采用一期或两期方式实施。无论采用何种技术,均应遵循外科与修复的一般原则。尽量少用电凝、行无张力缝合、选用血供良好的移植物或周围组织,并进行多层水密闭合,可最大限度减少常见潜在并发症。若尿道板存在且足够宽,TIP尿道成形术可作为一期修复的选择。Snodgrass和Lorenzo报道了在尿道下裂再修复中应用TIP尿道成形术的初步经验。他们共纳入15例患者,平均随访5个月;其中13例获得外观正常的尿道口,并发症包括2例瘘和1例龟头裂开。6 Shanberg及其同事报道了13例采用TIP进行再修复的病例,平均随访22个月。美容结果优异,但出现两例并发症:一例为龟头裂开并合并尿道皮肤瘘,另一例发生尿道口狭窄。20 对于明显瘢痕化且导致阴茎下弯的尿道板,应按Bracka倡导的两期修复方案将其切除,并以颊黏膜移植物替代。21
Bracka 将二期修复术在尿道下裂手术中的应用普及,作为对单期手术后高并发症发生率的一种潜在解决方案。他报道了文献中规模最大的系列,共600例(儿童457例,成人143例),其中34.8%为再次修复。总体而言,3.7%需要第一期翻修。在挽救性手术中,尿道皮肤瘘发生率为10.5%,而尿道狭窄发生率为7%。22 Hayes 等 报道在25例既往尿道下裂修复失败的患者(包括成人和儿童)中,于多期修复中采用颊黏膜移植物。22%需要第一期翻修,而仅1例(4%)发生尿道皮肤瘘。23 Barbagli 等 研究了60例继发性尿道下裂成人患者,其中31例接受了多期修复。颊黏膜移植在单期手术中的成功率为82%,在多期手术中的成功率亦为82%。单期与二期手术的尿道皮肤瘘发生率分别为10.3%和6.5%。5 Kulkarni 等 指出,在其采用 BMGs(颊黏膜移植)进行二期修复的病例系列中,颊黏膜移植物挛缩的发生率较高,这促使其将技术改为混合式方案。24
在大奥蒙德街儿童医院(GOSH),我们的两期修复结合了Turner-Warwick、Duplay和Cloutier历史上描述的技术。25 在第一期修复中,将瘢痕化的尿道沿正中向腹侧切开,直至健康的出血组织。围绕阴茎冠状沟完成包皮环切切口,常与既往切口重合,并在龟头近侧保留一圈黏膜袖口。随后切除龟头内的瘢痕区,并通过在正中做一深切口将龟头打开、铺开,然后自顶端将龟头组织分离抬起。在切除远端新尿道及瘢痕组织之前,将尿道延伸至管腔良好的健康组织区域。将失效的新尿道部分或全部切除,直至健康组织。通过使用止血带和注射生理盐水制造人工勃起以识别阴茎下弯,并通过腹侧松解瘢痕化新尿道,必要时联合背侧白膜褶皱缝合予以矫正。需要在近端形成较宽的尿道开口,并将阴茎干皮肤重新覆盖至阴茎背侧。这样会在腹侧从尿道开口到远端龟头尖以及皮肤边缘之间留下范围不等的生创面。若有多余的健康包皮皮肤,我们优先使用其作为移植物。但通常需要取用其他部位的移植组织,常见为耳后皮(Wolfe)移植、颊部内侧口腔黏膜移植,以及复合移植(口腔黏膜移植联合包皮内板)。取下选定的皮肤移植物后进行去脂处理,然后覆盖于阴茎腹侧的生创面。移植物通常呈矩形(至少2 cm宽),用6/0 Monocryl环周缝合,并用额外的quilting缝合将其固定于阴茎海绵体。使用卷起的Jelonet(石蜡纱布)将移植物压紧在阴茎干上,并通过在其上方将阴茎干皮肤边缘对合缝合来固定。随后以泡沫材料覆盖阴茎,并用外层Elastoplast胶带缠绕加压。根据患儿年龄选择使用滴流支架或持续引流导尿管(所有6岁以下患儿均使用滴流支架)。
第一周给予抗生素和抗胆碱药。去除敷料,并在全身麻醉下检查移植物。指导家长在移植物上每日两次涂抹氯霉素软膏,持续10天。三个月后在门诊复查,如果移植物令人满意,则计划进行第二期手术;若不满意,则使用一段时间的双氢睾酮软膏以进一步使移植物成熟并刺激阴茎生长。第二期手术最早不早于6个月进行,大多数病例在接近1年时修复。将尿道板和龟头围绕一根8 Fr导尿管缝合关闭,使用6-0 Monocryl缝线。随后再次应用泡沫敷料,并留置导尿管引流,同时给予抗生素和抗胆碱药物1周。之后由我们病房的护理人员在病房拆除敷料(无需任何镇静)。患者在门诊于3个月和12个月进行随访。
尿道皮肤瘘
尿道皮肤瘘是初次尿道下裂修复术后最常见的并发症之一,发生率为5%至15%。公认的风险因素包括修复手术年龄、血肿、移植物坏死、尿外渗、伤口感染以及术者经验。Duarsa 等 对591例患者进行的多中心回顾性研究得出结论:耻骨上引流可降低尿道下裂修复术后发生尿道皮肤瘘的风险。作者推测,经皮建立一过性尿路改道可减少尿液经新尿道的通过,并尽量降低组织反应、缝线活动度及感染风险。10 同时,还需排除远端新尿道口狭窄或阴茎腹侧皮肤缺血。手术处理将取决于尿道口大小、瘘的数量,以及是否存在合并性新尿道狭窄。Kulkarni 等 描述了其瘘修补技术:对于尿道口狭窄并伴有尿道狭窄的病例,从腹侧切开尿道直至见到满意口径的尿道。若尿道板大于8 mm,则行 Asopa 单期背侧嵌入式移植增量尿道成形术;若宽度小于8 mm,则采用两期法将其管状化以构建新尿道。最后,若尿道口径良好且无远端梗阻,可对尿道皮肤瘘行原发性关闭。24
复发性腹侧阴茎下弯
与腹侧阴茎延长术相比,接受过背侧褶缩缝合术的患者更常观察到腹侧阴茎弯曲(VC)。另一促成因素是瘢痕化腹侧皮肤及尿道周围纤维组织的挛缩。Flynn 等人 报告了青春期后尿道下裂修复术后复发性阴茎弯曲的迟发出现。他们的大多数患者报告,阴茎弯曲与青春期阴茎的生长相关。他们推测,复发性VC,尤其在青少年中,是由于海绵体壁不对称所致,这可能继发于发育不全的腹侧海绵体壁或重建尿道的不成比例生长。26 短的腹侧尿道与海绵体组织之间的不匹配是复发性腹侧弯曲的关键因素。27 在多数情况下,’轻度’(小于30度)的弯曲可通过皮肤松解或切断尿道板加以矫正。尿道下裂复修中的多数患者需要处理潜在的海绵体不均衡,基本有两种选择:要么缩短长侧,要么延长短侧。在长度宝贵的尿道下裂患者中,最好避免背侧褶缩缝合术。对复发性弯曲,优选腹侧横向海绵体切开术,可获得合理程度的矫正。对于弯曲超过30度的病例,可考虑分期修复,首先切除尿道,在海绵体上置入真皮移植,并以达尔托斯筋膜瓣和局部皮瓣覆盖。24
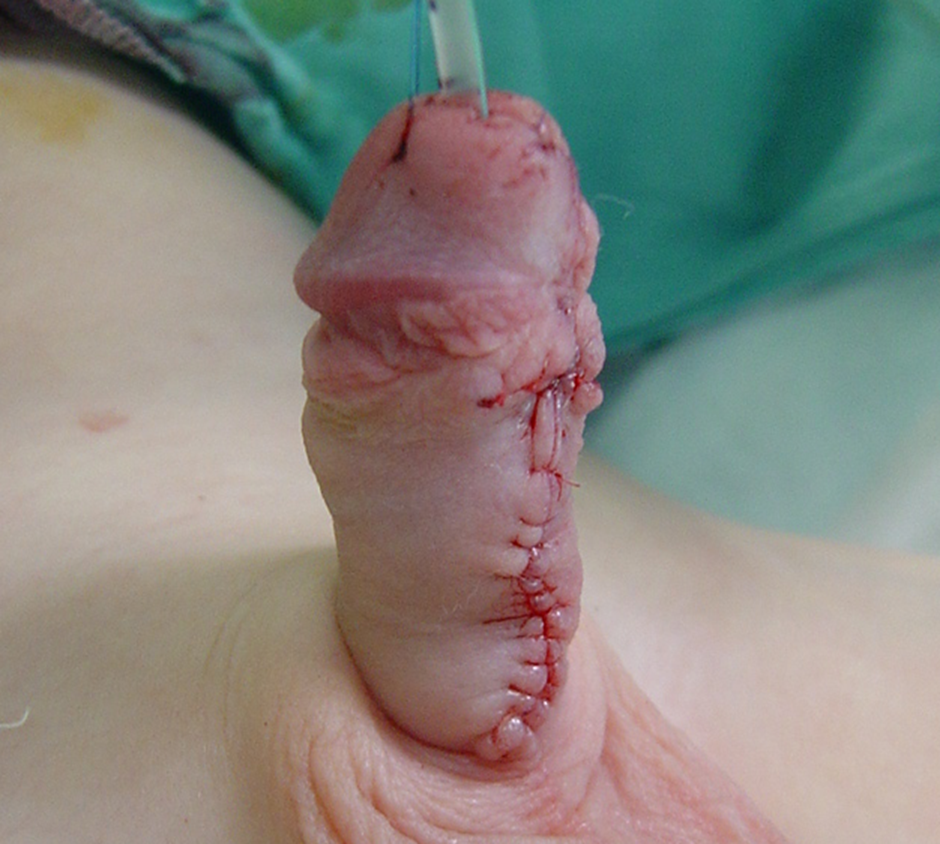
图5 在2期修复结束时完成管状化后的最终外观。导尿管将留置七天,随后在病房拆除敷料。
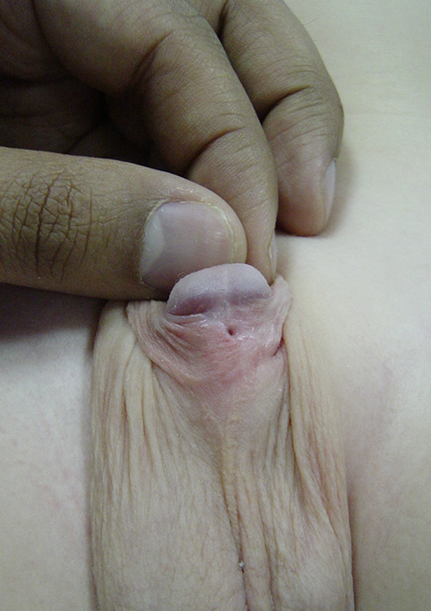
图 6 尿道下裂修复术后出现孤立性尿道皮瘘。适合行简单的瘘道切除与缝合关闭
术后护理
在 GOSH,继发性尿道下裂修复术作为一日住院手术进行,患儿术后过夜观察,次日出院。我们为所有男孩使用软性 8-French 喂养管进行导尿,并用 4-0 Prolene 暂留缝线将其固定于龟头,留置 7 天。封闭的环绕式敷料通过促进制动、手术部位保护、组织贴合和加压来优化术后愈合。目前,对于哪种敷料最有效,或是否需要应用敷料,尚无共识。一项随机试验发现,有无敷料组在并发症发生率或临床结局方面无显著差异。值得注意的是,无敷料组家长的术后电话明显增多。28 按照前述敷料技术,对于尚未进行如厕训练的儿童,我们使用双层纸尿裤。导尿管和敷料于 7 天后去除;对于伴移植的第一期修复,在全身麻醉下去除;第二期修复后则在病房局部去除。年龄较大的儿童使用 Foley 导尿管并连接引流袋。我们根据本地微生物学政策开具口服抗生素,并给予奥昔布宁 0.2 g/kg 每日一次,直至拔除导尿管,以预防膀胱痉挛。术前骶管神经阻滞有助于减轻术后疼痛。29 进一步的疼痛控制可通过小剂量 NSAID(非甾体抗炎药)镇痛药实现。
结论
尿道下裂再手术对于积极照护尿道下裂患儿的外科医生而言,在治疗与管理上具有较高的手术挑战性。由于其并发症率低且技术具有可重复性,作者主张对尿道下裂再手术采用两期修复。尽管如此,若在尽最大努力后仍然失败,可考虑会阴尿道造口术,以维持这些患者的生活质量。现有文献中针对尿道下裂再手术外科处理各方面的高质量证据有限。因此,应当鼓励开展更多研究以改善患者结局。下面概述了一个用于尿道下裂再手术外科处理的简明流程。
图 7 尿道下裂再手术或复修的建议管理算法。
家庭资源
推荐阅读
- Shukla AR, Patel RP, Canning DA. Hypospadias. J Pediatr Urol: 103–126. DOI: 10.1385/1-59259-421-2:103.
- Chapter 15: Hypospadia Paediatric Urology WebBook, European Society of Paediatric Urology. 20AD: 227–256.
参考文献
- Butwicka A, Lichenstein P, Landen M. Hypospadias and increased risk for neurodevelopmental disorders. Child Psychol Psychiatry 2015; 56 (2): 155–161.
- Jin TT, Wu WZ, Shen ML. Hypospadias and Increased Risk for Psychiatric Symptoms in Both Childhood and Adolescence: A Literature Review. Front Psychiatry 2022; 13: 799335. DOI: 10.3389/fpsyt.2022.799335.
- Craig W JR, C B, WO H, JM M, J.B.. Management of adults with prior failed hypospadias surgery. 2014; 3 (2): 196–204.
- Mundy AR. Failed hypospadias repair presenting in adults. Eur Urol 2006 (5): 774–776.
- Barbagli G, Perovic S, Djinovic R, Sansalone S, Lazzeri M. Retrospective descriptive analysis of 1,176 patients with failed hypospadias repair. J Urol 2010 (1): 207–211. DOI: 10.1016/s0084-4071(10)79536-1.
- Snodgrass WT. A Lorenzo Tubularized incised plate for hypospadias reoperation. BJU Int 2002; 89: 98–100. DOI: 10.1097/01.ju.0000125018.90605.a5.
- Al-Sayyad A, Pike JG, Leonard MP. Redo hypospadias repair: experience at a tertiary care children’s hospital. Can Urol Assoc J 2007; 1 (1). DOI: 10.5489/cuaj.39.
- Duarsa GWK, Tirtayasa PMW. Risk factors for urethrocutaneous fistula following hypospadias repair surgery in Indonesia. J Pediatr Urol 2020; 16 (3): 317.e1–317. DOI: 10.1016/j.jpurol.2020.04.011.
- Wilkinson DJ, Green PA, Beglinger S. Hypospadias surgery in England: Higher volume centres have lower complication rates. J Pediatr Urol 2017; 13 (5): 481.e1–481.e6.
- Fichtner J, Filipas D, Mottrie AM, Voges GE, Hohenfellner R. Analysis of Meatal Location in 500 Men: Wide Variation Questions Need for Meatal Advancement in All Pediatric Anterior Hypospadias Cases. J Urol 1995; 154 (2): 833–834. DOI: 10.1016/s0022-5347(01)67177-5.
- Dodds PR, Batter SJ, Shield DE, Serels SR, Garafalo FA, Maloney PK. Adaptation of Adults to Uncorrected Hypospadias. Urology 2008; 71 (4): 682–685. DOI: 10.1016/j.urology.2007.07.078.
- Chang C, White C, Katz A, Hanna MK. Management of ischemic tissues and skin flaps in Re-Operative and complex hypospadias repair using vasodilators and hyperbaric oxygen. J Pediatr Urol 2020; 16 (5): 672.e1–672.e8. DOI: 10.1016/j.jpurol.2020.07.034.
- Wright I, Cole E, Farrokhyar F, Pemberton J, Lorenzo AJ, Braga LH. Effect of Preoperative Hormonal Stimulation on Postoperative Complication Rates After Proximal Hypospadias Repair: A Systematic Review. J Urol 2013; 190 (2): 652–660. DOI: 10.1016/j.juro.2013.02.3234.
- Smith J, Patel A, Zamilpa I. Commentary to ‘Is parenteral antibiotic prophylaxis associated with fewer infectious complications stented, distal hypospadias repair?’ J Pediatr Urol 2017; 18 (6): 764. DOI: 10.1016/j.jpurol.2022.05.024.
- Baillargeon E, Duan K, Brzezinski A, Jednak R, El-Sherbiny M. The role of preoperative prophylactic antibiotics in hypospadias repair. Can Urol Assoc J 2014; 8 (7-8): 236. DOI: 10.5489/cuaj.1838.
- Hsieh MH, Wildenfels P, Gonzales ET. Surgical antibiotic practices among pediatric urologists in the United States. J Pediatr Urol 2011; 7 (2): 192–197. DOI: 10.1016/j.jpurol.2010.05.001.
- Chua ME, Kim JK, Rivera KC. Commentary to ‘The use of postoperative prophylactic antibiotics in stented distal hypospadias repair: a systematic review and meta-analysis.’ J Pediatr Urol 2019; 15 (2): 149. DOI: 10.1016/j.jpurol.2018.10.024.
- Zhu C, Wei R, Tong Y, Liu J, Song Z, Zhang S. Analgesic efficacy and impact of caudal block on surgical complications of hypospadias repair: a systematic review and meta-analysis. Reg Anesth Pain Med 2019; 44 (2): 259–267. DOI: 10.1136/rapm-2018-000022.
- Tanseco PP, Randhawa H, Chua ME, Blankstein U, Kim JK, McGrath M, et al.. Postoperative complications of hypospadias repair in patients receiving caudal block vs. non-caudal anesthesia: A meta-analysis. Can Urol Assoc J 2019; 13 (8): 249–257. DOI: 10.5489/cuaj.5688.
- Retik AB, Borer JG. Primary and Reoperative Hypospadias Repair With the Snodgrass Technique. J Urol 1998; 16 (3): 1561. DOI: 10.1097/00005392-199910000-00122.
- Bracka A. Hypospadias repair: the two-stage alternative. Br J. Br J Urol 1995; 76: 31–41. DOI: 10.1111/j.1464-410x.1995.tb07819.x.
- Bracka A. The role of two-stage repair in modern hypospadiology. Indian J Urol 2008; 24 (2): 210–218.
- Hayes MC, Malone PS. The use of a dorsal buccal mucosal graft with urethral plate incision (Snodgrass) for hypospadias salvage. BJU Int 1999; 83 (4): 508–509. DOI: 10.1046/j.1464-410x.1999.00043.x.
- Kulkarni S, Joglekar O, Alkandari M, Joshi P. Redo hypospadias surgery: current and novel techniques. Res Rep Urol 2018; Volume 10: 117–126. DOI: 10.2147/rru.s142989.
- S JN, T N, K OM, M CP. The two-stage repair for severe primary hypospadias. Eur Urol 2006; 50 (2): 366–371.
- Flynn JT, Johnston SR, Blandy JP. Late Sequelae of Hypospadias Repair. Br J Urol 1980; 52 (6): 555–559. DOI: 10.1111/j.1464-410x.1980.tb03114.x.
- Abosena W, Talab SS. Moneer K Hanna Recurrent chordee in 59 adolescents and young adults following childhood hypospadias repair. J Pediatr Urol 2020; 162 (e1-162.e5). DOI: 10.1016/j.jpurol.2019.11.013.
- Van Savage JG, Palanca LG, Slaughenhoupt BL. A Prospective Randomized Trial Of Dressings Versus No Dressings For Hypospadias Repair. J Urol 2000: 981–983. DOI: 10.1097/00005392-200009020-00015.
- O’Kelly F, Pokarowski M, DeCotiis KN, McDonnell C, Milford K, Koyle MA. Structured opioid-free protocol following outpatient hypospadias repair - A prospective SQUIRE 2.0-compliant quality improvement initiative. J Pediatr Urol 2020; 16 (5): 647.e1–647.e9. DOI: 10.1016/j.jpurol.2020.06.012.
- Shukla AR, Patel RP, Canning DA. Hypospadias. J Pediatr Urol: 103–126. DOI: 10.1385/1-59259-421-2:103.
- Chapter 15: Hypospadia Paediatric Urology WebBook, European Society of Paediatric Urology. 20AD: 227–256.
最近更新时间: 2025-09-22 08:00