18: 憩室、尿囊异常与前列腺小囊
阅读本章大约需要 9 分钟。
脐尿管异常
胚胎学与解剖学
脐尿管是一种管状结构,将位于脐部的尿囊与膀胱顶相连,并在妊娠期保持通畅。1 管腔通常在妊娠约第12周闭合,并完全闭锁。闭锁后,通常仅残留一条自脐部下方通向膀胱顶的纤维索。脐尿管位于腹膜外,在腹腔镜下观察骨盆时易于显露 (图 1).
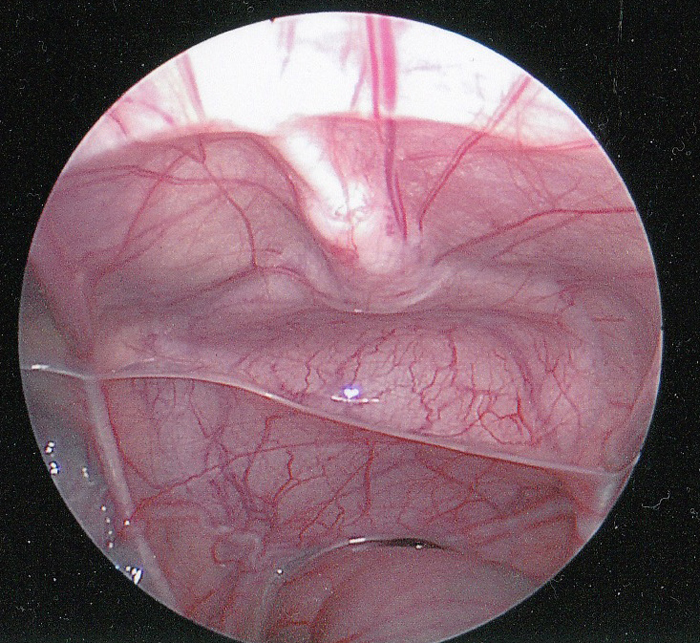
图 1 尿膜管的腹腔镜腹腔内视图。尿膜管的纤维索(正中脐韧带)可见位于正中线,向下延伸至膀胱顶。直肠位于下方。可见输精管在直肠上方向外侧延伸。
尿膜管被腹膜的皱襞所覆盖,形成正中脐韧带。罕见情况下,尿膜管的纤维索可能存在间断,甚至索条完全闭锁。它是用于标示膀胱穹隆部的重要外科标志,以确保膀胱造口术的正确放置。
尿囊管异常的分类
脐尿管异常是由于胚胎发育过程中脐尿管管腔未能完全闭锁所致。2 其解剖学分型基于脐尿管通畅持续的程度,范围可从完全通畅并有尿液自由流出,到自皮肤起始、以盲端结束的小窦道(图 2)。脐尿管囊肿可位于脐尿管全程的任何部位,但最常见于膀胱顶附近。脐尿管憩室—所报道的最罕见异常—是脐尿管部分通畅,向膀胱顶引流。
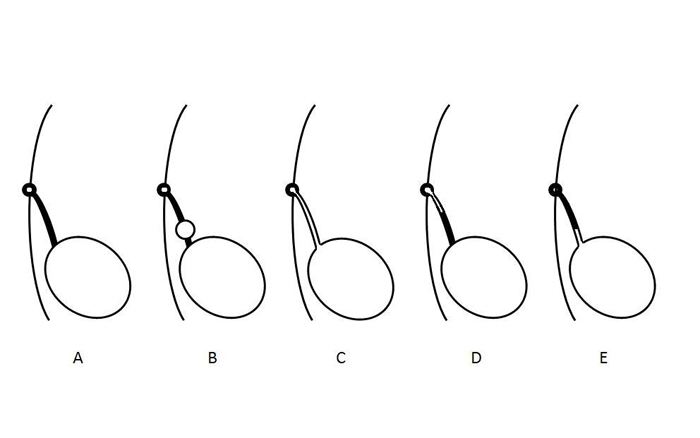
图2 示意图说明尿膜管异常的不同类型。A- 尿膜管腔的正常闭锁。B- 尿膜管囊肿。C- 尿膜管未闭。D- 尿膜管窦。E- 尿膜管憩室。这些异常并非相互排斥,可能以组合形式同时出现。
来自若干临床系列的不同类型脐尿管异常的相对发生率见表 1.3,4,5,6,7,8,9
表 1 儿童脐尿管异常类型及发生率概述。
| 作者 | 病例数 | 囊肿 | 开放性 | 窦道 | 憩室 |
|---|---|---|---|---|---|
| Naiditch | 103 | 38 | 21 | 11 | 13 |
| Fox | 66 | 34 | 14 | 7 | 10 |
| Ashley | 46 | 25 | 6 | 14 | 1 |
| Cilento | 45 | 16 | 7 | 22 | 0 |
| Rich | 35 | 12 | 19 | 4 | 0 |
| Yiee | 31 | 19 | 7 | 5 | 0 |
| Copp | 29 | 7 | 3 | 17 | 2 |
| 总计 | 355 | 151 (43) | 108 (30) | 80 (23) | 26 (7) |
有趣的是,仅有一例关于尿膜管未闭的病例报告,在导尿管引流2周后自行闭合,并残留为尿膜管憩室。10
临床表现
脐尿管异常患儿最常见的就诊表现为产后持续数周的脐部渗出,或因感染导致的肿块和/或疼痛。3,6,8,11 脐部分泌物可为清亮、浆液性、脓性或血性,并可提示其病因:婴儿持续有清亮液体(很可能为尿液)漏出提示脐尿管未闭,而混浊、浆液性或血性液体提示脐尿管窦道或囊肿。其发病年龄呈双峰分布,脐尿管窦道或未闭者的平均就诊年龄为1-3个月,而以脐尿管囊肿就诊者为3岁。8 脐部渗出的鉴别诊断还包括脐炎、脐肠管残余或脐肉芽肿.6
表 2 脐尿管异常患儿的就诊症状。
| 作者 | 病例数 | 引流 | 肿块/感染 | 疼痛 | 无症状 | 其他 |
|---|---|---|---|---|---|---|
| Gleason | 721 | 26 | 19 | 17 | 667 | 0 |
| Naiditch | 103 | 60 | 7 | 4 | 18 | 12 |
| Stopak | 85 | 43 | 36 | 0 | 4 | 1 |
| Dethlefs | 68 | 52 | 32 | 0 | 8 | 0 |
| Cilento | 45 | 19 | 15 | 10 | 0 | 1 |
| Yiee | 37 | 20 | 8 | 4 | 2 | 3 |
| 合计 | 1059 | 220 (21) | 117 (11) | 35 (3) | 699 (66) | 17 (2) |
体格检查也可能有帮助。 尿膜管未闭或尿膜管窦可表现为脐底部的小凹或凹痕 (图3).
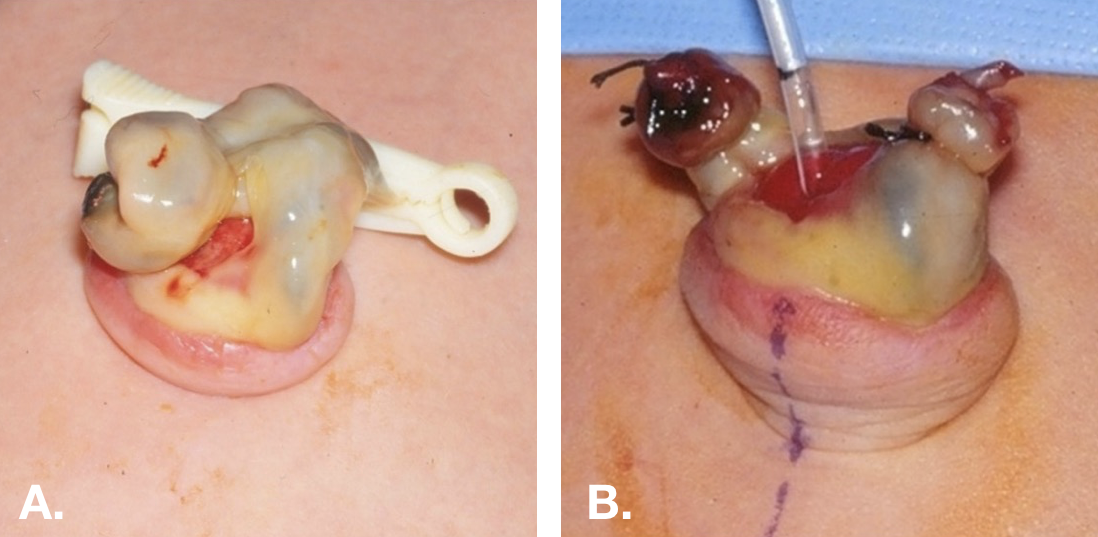
图 3 新生儿的尿脐管未闭。A. 显示尿脐管未闭的脐端呈牛肉样鲜红外观。B. 将脐部皮肤外翻,并经尿脐管未闭处送入一根小号喂养管至膀胱。
一些尿膜管异常是在对其他疾病(如尿路感染或产前肾积水)进行常规影像学评估时偶然发现的。尿膜管囊肿常可在膀胱超声检查时被发现(图 4)。
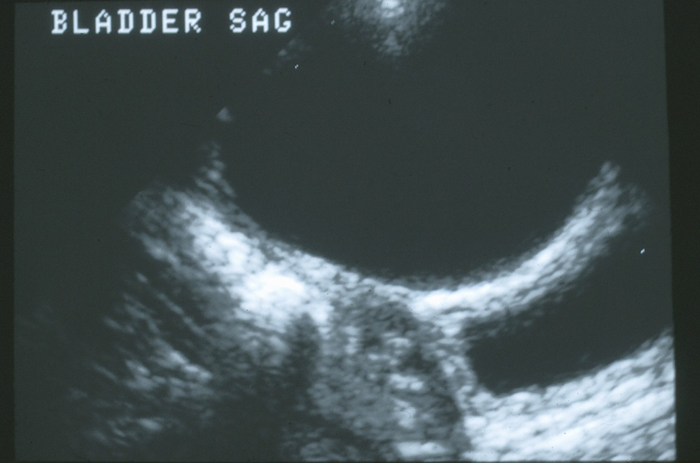
图 4 中线处膀胱穹隆的矢状位图像。膀胱壁前方可见一个大的无回声脐尿管囊肿。
尿囊癌
由于脐尿管癌并非有报道在儿童或青少年中出现的疾病,本章将不包括该疾病的治疗。5,12 如将在管理部分讨论的那样,无症状的脐尿管异常发展为癌的风险,以及因此进行预防性切除的价值,尚不明确。 脐尿管异常发生癌变的风险因素包括病变大小大于 4 cm 以及年龄 > 55 岁。钙化是癌的常见特征,然而,在无症状的良性脐尿管残余中出现钙化的意义尚不清楚。5,12 鉴于钙化可能与慢性炎症相关,而慢性炎症与致癌相关,切除伴有钙化的病变似乎是谨慎的做法。
影像学评估
影像学检查的选择取决于就诊时的症状以及临床怀疑的程度。6,8 对于允许尿液经脐部自由引流的尿膜管未闭,可通过排尿性膀胱尿道造影 (VCUG) 或窦道造影获得高度敏感的影像学显示。传统上采用 VCUG 来识别尿膜管未闭,同时对膀胱进行解剖学评估,并评估是否存在膀胱出口梗阻或膀胱输尿管返流 (图 5). 然而,腹部超声对大多数患者的诊断已足够、更为普及、创伤更小且费用更低;因此,它已成为诊断的标准,而 VCUG 则保留用于复杂病例。3,13 应告知超声医师对尿膜管异常的临床怀疑,以便对腹壁正中进行彻底检查。感染性尿膜管囊肿表现为伴有复杂液体集合的大型异质性肿块 (图 6).
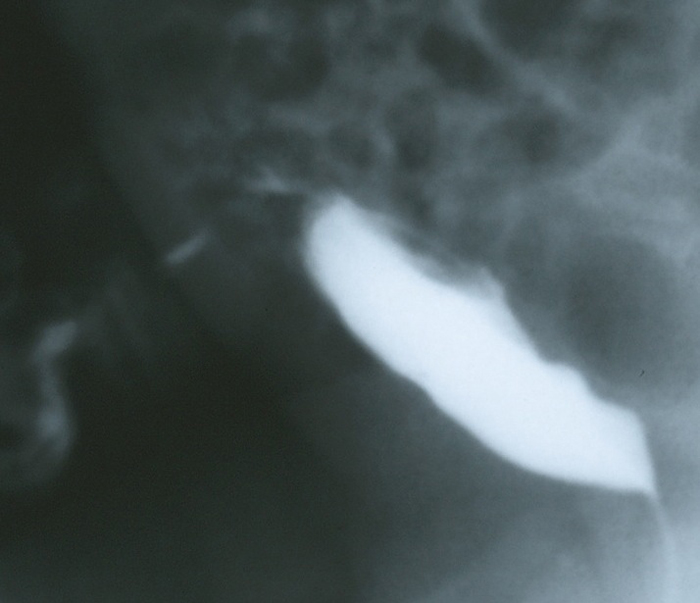
图 5 一名脐部有大量清亮液体流出的婴儿的 VCUG 侧位充盈相。图像显示造影剂经通畅的尿囊管从膀胱穹隆流经前腹壁引流。
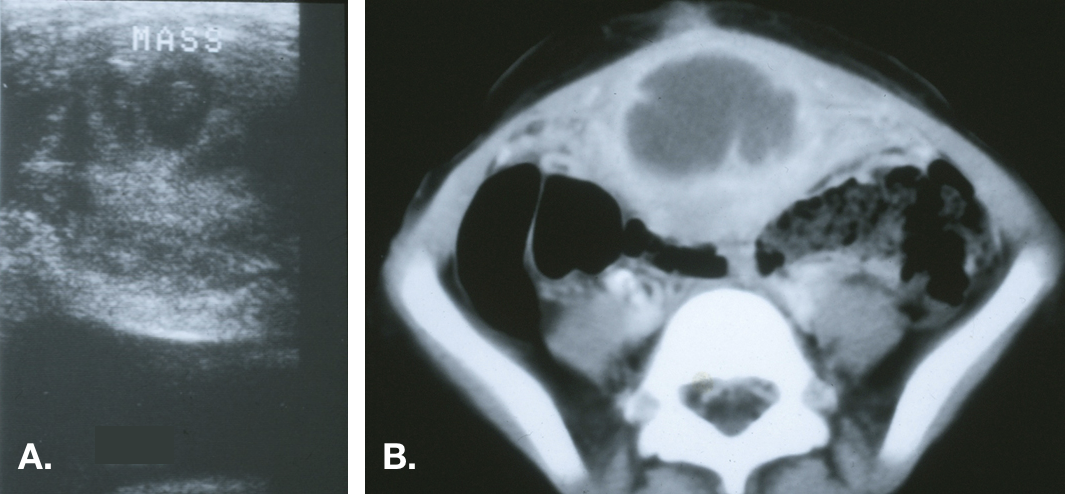
图 6 感染性尿囊囊肿。A. 中线处膀胱穹隆的横断面,显示膀胱上方的不均匀回声肿块。B. CT 显示中线肿块中央为液体密度,与脓肿相符,并见邻近前腹壁的炎性条索影。
这些病变的直径可达数厘米,并且在某些情况下可向腹膜前间隙之外扩展,甚至穿破进入腹腔。14,15
CT 不应被视为常规检查中的必要组成部分,但可发现超声 (US) 漏诊的异常,因此在临床高度怀疑但缺乏明确诊断的病例中有用 (图 7).
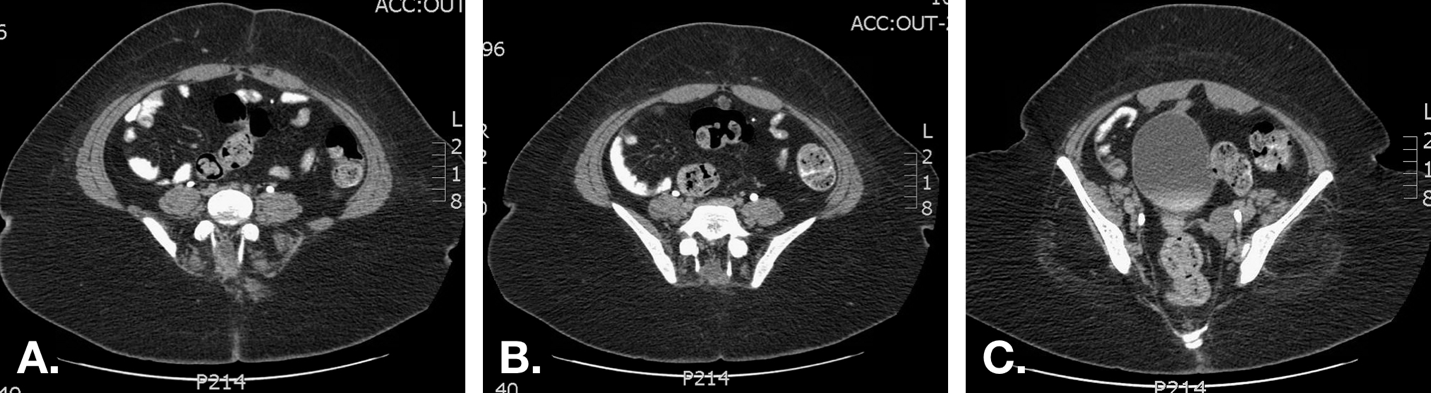
图7 有反复血性脐部流出物患者的CT扫描。A. 脐部稍下方的图像显示正中线腹直肌下方可见外观正常的尿囊(纤维索带)。B. 更向下方可见尿囊囊肿。C. 显示囊肿下端与膀胱顶的邻近关系。
评估还应包括肾脏超声检查,以确保不存在肾积水或其他先天性肾脏异常.6,8,9,16 已发表病例系列中并存肾脏异常的发生率差异很大,但鉴于超声检查缺乏相关不良影响和风险,将对肾脏进行影像学检查纳入评估方案的一部分是明智之举。4
治疗
一般而言,有症状的脐尿管残余应行手术切除。这应包括自脐部至膀胱顶的脐尿管的完全切除(图 8)。
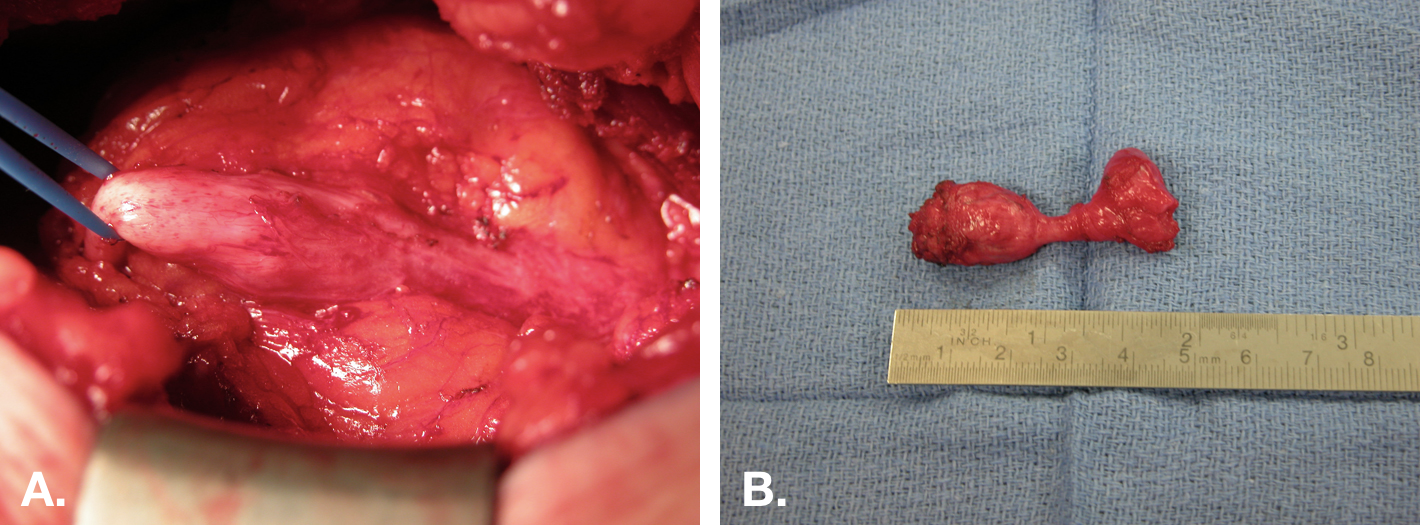
图 8 脐尿管囊肿的开放切除。A. 通过脐下正中切口行脐尿管囊肿切除的术中视图。囊肿位于中央,右侧为与膀胱的附着,左侧为向脐部延续的纤维索。B. 切除后的标本(囊肿),带有一小块膀胱袖口(标本右端)。
对于与膀胱壁不相通的尿囊囊肿或尿囊窦,是否需要切除膀胱黏膜,尚存争议。2,6,7,17,18 感染的尿囊囊肿则提出了另一难题,因为感染导致囊肿明显增大,且周围炎症使单纯切除更为困难,并可能增加并发症风险。可以对感染的尿囊囊肿采取单期或分期处理。7,17,19 下文将进一步讨论这两种方法的相对优点与风险。
手术切除尿囊残余具有治愈性,且由于其为退化的残余结构,切除后不会产生功能性后遗症。主要的手术决策困境出现在影像学偶然发现无症状病灶的患者。
保守治疗
关于原位保留的脐尿管异常之长期后遗症—即复发性感染和癌的发生—的数据有限。鉴于推定的恶变风险,历史上更倾向于行切除,然而,近期文献主张采取保守策略.10,20 已有报道,即使在存在感染的情况下,脐尿管未闭可闭合,脐尿管囊肿可消退.21,22,23 感染囊肿经皮引流后症状复发似乎较为罕见.23 在一项病例系列中,接受观察的< 6月龄婴儿中有92%感染消退,而接受切除者有60%发生术后感染,这提示至少至6月龄之前采取保守管理可能更为稳妥.21
对于感染性尿膜管囊肿,应进行尿培养以及(如有)伤口引流液培养。感染性尿膜管囊肿中最常培养出的致病菌是金黄色葡萄球菌(表 3)。5,21,18,20 若患者病情稳定、无发热、无中毒表现、无腹膜刺激征、无蔓延性蜂窝织炎或筋膜炎征象,则可考虑初始仅用抗生素进行治疗。
表 3 从感染的脐尿管残余中培养出的微生物种类。 * 大肠杆菌-3、柠檬酸杆菌、肠球菌和变形杆菌。
| 作者 | 患者数 | 金黄色葡萄球菌 | 链球菌属 | 其他 * |
|---|---|---|---|---|
| Stopak | 16 | 9 | 0 | 2 |
| McCollum | 9 | 6 | 1 | 2 |
| Minevich | 9 | 9 | 0 | 0 |
| Ashley | 9 | 6 | 1 | 2 |
| Galati | 5 | 5 | 0 | 0 |
| Newman | 5 | 3 | 0 | 2 |
| 合计 | 53 | 38 (72) | 2 (4) | 8 (15) |
对于病情不稳定或保守治疗失败的患者,感染性囊肿可通过初始完全手术切除,或经皮(在超声或CT引导下)或开放引流进行处理。对感染性脐尿管进行单期切除时,切除边界通常更大,导致更大的软组织缺损;且炎症范围可能延伸至腹腔内,从而使腹腔脏器面临风险。与引流相比,对感染性脐尿管囊肿行一期切除后,发生肠皮瘘的风险虽小但具有临床意义,且伤口并发症的发生率更高。21,18,19,24 经皮引流可能无法完全引流多房性液体集合。传统上,待炎症消退后以分期方式切除所有囊肿。近期的多项研究显示,复发性感染并不常见,因此未必需要延迟行切除术。
腹腔镜下脐尿管切除术
在微创外科时代,已有多篇关于在儿童中开展腹腔镜以及更近期的机器人辅助腹腔镜脐尿管残余切除术的报道。25,26,27 亦有单孔腹腔镜手术的报道。28 与纯腹腔镜相比,机器人技术的主要优势在于缝合更为容易。27 采用腹腔镜途径对膀胱的视野极佳,但若穿刺孔未谨慎放置,在脐部的视野可能更具挑战。 脐尿管应自脐底至膀胱顶予以切除。 再者,关于完全切除是否需要切除膀胱顶仍存在争议。25,26
孔位放置是一个重要的考虑因素。26 由于脐尿管起源于脐部,此处不能作为孔位. 最常见的镜孔位于脐上(通常为1–2 cm)。这可提供足够的操作空间,以观察从脐部到膀胱顶的分离。工作孔应置于两侧,通常在脐平面。或者,将侧方工作孔与镜孔(置于腹部右侧或左侧)进行布置,可提供对脐尿管的侧方观察,并使所有孔位均位于脐下。由于角度关系,这种侧方孔位配置会使膀胱闭合的缝合更为困难。

图 9 脐尿管残余切除术可能的套管孔(穿刺孔)布置示意图。与大多数腹腔镜手术的布置不同,脐部不能作为穿刺孔位点,因为它是脐尿管的起始部,且在解剖分离过程中必须予以直视显露。
尿囊管开放切除术
在婴儿和年幼儿童中,可通过小切口轻松完成尿膜管的完全切除。切口方向可为横向或正中纵向。对于婴儿,在耻骨与脐之间的中点处做一个1–1.5 cm的小切口,即可进入尿膜管,并可自脐部至膀胱穹隆行完全切除,同时可充分暴露膀胱穹隆以便关闭。该小切口的大小与手术机器人12 mm摄像镜穿刺口所需的切口相当,并可使手术全程保持腹膜外,避免潜在的腹腔内并发症。对于年龄较大或肥胖的儿童或青少年,宜选择正中纵形切口。若暴露困难,可将切口向上延至脐部或向下延至膀胱,以利于在这些患者中完全切除尿膜管。单纯切除的并发症发生率很低,手术可在门诊完成;若留置导尿管,则可短期住院。尿漏或切口并发症更常见于对感染性囊肿进行的一期切除,而非单纯切除。18,19
要点
- 尿膜管异常最常见的表现是分娩后持续数周的脐部渗出,或因感染导致的脐部肿块和/或疼痛。
- 脐部渗出的鉴别诊断包括尿膜管异常、脐炎、脐肠管残余,或脐肉芽肿。
- 无症状尿膜管异常发生癌变的绝对风险尚不明确。尿膜管异常发生癌变的危险因素包括病变大小大于4 cm以及年龄>55岁。
- 腹部超声在多数患者中足以用于诊断尿膜管异常。检查应包括肾脏超声以评估先天性肾脏畸形。
- 有症状的尿膜管残端应经开放手术、腹腔镜或机器人辅助手术切除。若采用腹腔镜或机器人入路,脐部不能作为穿刺孔位。
- 无并发症的尿膜管囊肿感染可保守治疗,使用抗生素±经皮引流。立即切除具有显著的术后感染风险。延期切除是合理的,但可能并非必要,因为残端/囊肿有可能闭合,且复发感染较为少见,尤其在<6个月的儿童中。
前列腺小囊
胚胎学与解剖学
在男性胎儿中,塞尔托利细胞产生的穆勒管抑制物(MIS)在妊娠第8-10th周促使副中肾(穆勒)管迅速退化—这些结构在女性中将发育为输卵管、卵管、子宫和阴道的上2/3—。前列腺小囊是副中肾管的一个小残余,在男性中作为前列腺尿道的一个扩张而持续存在。1 该小囊起源于精阜头侧面的前列腺正中线。
临床表现
除非其完全退化受阻并使小囊保持扩张、呈囊性外观,表现为前列腺部尿道的憩室,否则前列腺小囊(PU)并无临床意义。小囊增大与尿道下裂、隐睾、性发育障碍以及单侧肾缺如相关。29,30,31,32,33,34 病例系列显示,在近端尿道下裂患儿中,PU 扩张发生于 10-33% 的患者,且其发生率随尿道下裂缺陷严重程度增加而升高。30,32 在一项包含外生殖器外观正常但存在症状性 PU 的男孩的系列研究中,近三分之一的病例发现有单侧肾缺如。33
PU 可能无症状,可引起与梗阻或尿淤滞相关的症状—复发性尿路感染、附睾-睾丸炎、尿道分泌物、尿潴留、结石形成,或排尿后滴漏—或可表现为腹痛、血尿或血精.31,33,35 罕见情况下,已有恶性转化的报道。33,34,36
前列腺小囊的扩张可能妨碍尿道下裂男性的导尿—在这些患者中,导尿困难可能是前列腺小囊的首发表现或唯一症状。体积较大的前列腺小囊可在直肠指检时于膀胱的下方或后方触及。31,33,35,37
前列腺小囊的鉴别诊断包括穆勒管囊肿(不与前列腺部尿道相通)、膀胱憩室、尿膜管囊肿和精囊囊肿。33,38
表 4 合并前列腺子宫的患者的就诊时症状
| 作者 | 例数 | 尿路感染 | 下尿路症状 | 尿潴留 | 附睾炎/ 阴囊疼痛 | 其他 |
|---|---|---|---|---|---|---|
| Desautel | 26 | 6 | 1 | 7 | 2 | 1 |
| Liu | 22 | 8 | 7 | 6 | 3 | 6 |
| Dai | 15 | 6 | 2 | 1 | 7 | 4 |
| Jia | 14 | 8 | 1 | 1 | 5 | 3 |
| Babu | 7 | 3 | 2 | 1 | 3 | 1 |
| 合计 | 84 | 31 (37) | 13 (15) | 16 (19) | 20 (24) | 5 (6) |
影像学评估
诊断通常通过逆行尿道造影(RUG)或排尿性膀胱尿道造影(VCUG)确立,因为这些检查能够显示前列腺小囊开口与尿道和膀胱的关系。 然而,经腹超声的敏感性几乎与RUG相当,且足以检出大到需要干预的前列腺小囊。30,39 由于充盈不全,前列腺小囊可能在影像学检查中被漏诊。可以进行横断面影像学检查并有助于手术规划,但对诊断并非必需。可在影像学上对前列腺小囊进行如下分型:29
- 0 级 – 局限于精阜
- 1 级 – 延伸至膀胱颈或其以下 (图 1)
- 2 级 – 延伸至膀胱颈的近端 (图 2)
- 3 级 – 开口位于外尿道括约肌的远端
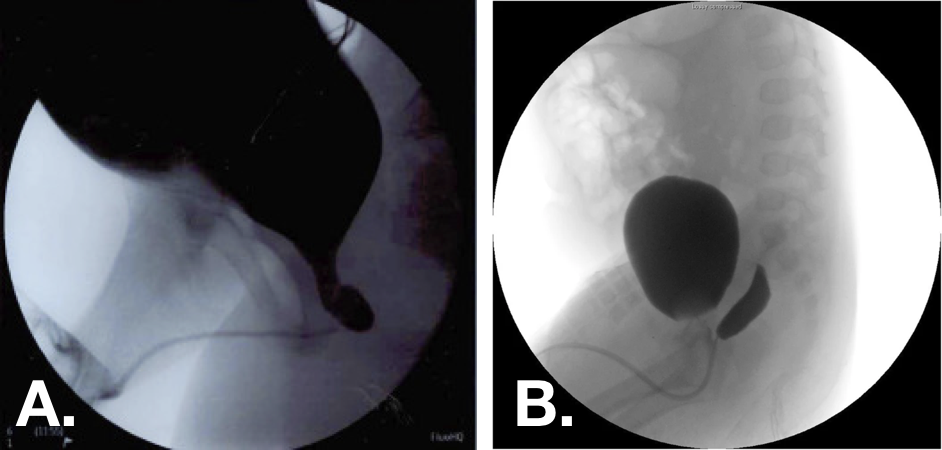
图 10 排尿期膀胱尿道造影(VCUG)图像,显示前列腺小囊1级(左)和2级(右)。30,33
处理
对于有症状的前列腺小囊,成功的保守治疗尚缺乏充分描述,且通常需要为反复感染进行频繁或长期的预防性抗生素治疗。33 已报道多种成功的手术方式,其中对前列腺小囊的完全切除可提供根治性处理。 对于开口极小的前列腺小囊,已有经直肠超声引导下抽吸、经尿道置管及抽吸、开口切开以及电灼术的报道。31,33,35,40 这些方法为微创,但较切除术的症状复发率更高。开放、腹腔镜及机器人辅助的切除术入路均已有报道,且关于最佳入路尚无共识。 由于前列腺小囊的位置较难到达,机器人入路可能具有优势。前列腺小囊可经腹膜外、膀胱外、经膀胱、后正中矢状或会阴入路到达。31,33,35,37,41 同时行膀胱镜检查可帮助在腹腔镜手术中明确解剖分离的界限。37,41 据报道,腹腔镜行前列腺小囊切除术转为开放手术的情况并不常见。
无论采取何种入路,切除的关键原则是充分剥离与切除,对前列腺小囊开口进行水密缝合,并避免损伤骨盆神经、输精管、精囊、精阜及直肠。
表 5 前列腺小囊切除的手术途径及术后就诊症状的复发
| 手术方式 | 内镜 | 内镜 | 开放 | 开放 | 腹腔镜 | 腹腔镜 |
|---|---|---|---|---|---|---|
| 作者 | 例数 | 复发 | 例数 | 复发 | 例数 | 复发 |
| Liu | 3 | 0 | 11 | 0 | 4 | 0 |
| Desautel | 2 | 1 | 13 | 0 | – | – |
| Dai | 13 | 5 | – | – | 4 | 0 |
| Jia | – | – | 8 | 2 | 6 | 0 |
| Babu | 7 | 2 | 2 | 2 | 1 | 1 |
| Yeung | – | – | – | – | 4 | 0 |
| Bayne | – | – | – | – | 4 | 0 |
| 总计 | 25 | 8 (32) | 34 | 4 (12) | 23 | 1 (4) |
要点
- 前列腺小室是副中肾管的一个小残余,在男性中作为前列腺尿道的一个膨大延伸而持续存在。
- 前列腺小室增大会与尿道下裂、隐睾、性分化异常以及单侧肾缺如相关。
- 前列腺小室可无症状,可导致与梗阻或尿淤积相关的症状,亦可表现为腹痛、血尿或血精。
- 前列腺小室的扩张可妨碍对患有尿道下裂的男性进行导尿。
- 有症状的前列腺小室可通过开放、腹腔镜或机器人辅助手术予以切除。
膀胱憩室
膀胱憩室在儿科人群中较为罕见,发生率为 1.7%。42 憩室可为先天性或后天性;在儿科人群中,先天性膀胱憩室更为常见。43
先天性憩室
先天性膀胱憩室被认为是由于逼尿肌缺陷所致。百分之九十发生于包含输尿管的 Waldeyer 鞘周围,称为 Hutch 憩室。44 可分为输尿管周围型(憩室靠近但不累及输尿管口)或旁输尿管型(憩室累及输尿管口)。44 膀胱壁及输尿管肌性隧道的变形使患有这些憩室的儿童易发生膀胱输尿管返流。与输尿管无关的先天性憩室多发生于膀胱后外侧。43
先天性膀胱憩室与影响结缔组织发育的遗传综合征有关:埃勒斯-当洛综合征 IV、V、IX 型;门克斯综合征;威廉姆斯-伯伦综合征;以及胎儿酒精综合征。41,44,45 与获得性憩室不同,先天性憩室见于膀胱壁光滑者,通常表现为正常的排尿动力学,为单发性缺陷,且不与恶性肿瘤风险增加相关。43 先天性憩室在男性更为常见,且通常比因神经源性膀胱功能障碍而获得的憩室更大。43
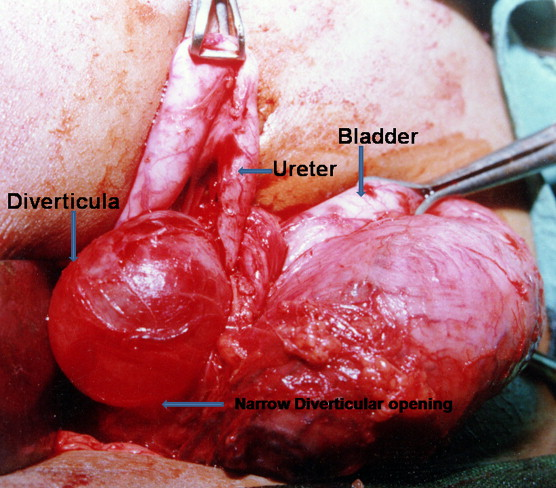
图 11 术中示大型输尿管旁膀胱憩室,伴输尿管近端扩张及内侧移位.46
后天性憩室
获得性或继发性憩室由于下尿路的神经源性或梗阻性病变而形成。尽管在成人中属于最常见的憩室类型,继发性憩室在儿科患者中却罕见,并提示下尿路严重功能障碍。43 它们更可能位于膀胱穹隆附近,可能为多发,且更常见于小梁化的膀胱。总体而言,获得性憩室与神经源性膀胱和膀胱出口梗阻相关,而在儿科患者中,可见于后尿道瓣膜及梅干腹综合征。42,43
临床表现
在儿童中,膀胱憩室最常表现为由于憩室内尿潴留继发的尿路感染,或在为肾积水或发热性尿路感染并怀疑膀胱输尿管反流而进行的检查过程中被发现.44,46 上尿路异常在16-30%的病例中可见。44 较少见的表现包括刺激性排尿症状、血尿、腹胀和腹痛、遗尿、膀胱结石,以及因膀胱颈变形导致的膀胱出口梗阻。46,47,48 罕见情况下,膀胱憩室可表现为完全性输尿管梗阻或腹膜内或腹膜外穿孔。49,50,51,52,53,54
大多数先天性膀胱憩室患者无症状,且—尽管这种情况远不如获得性憩室常见—也可能在成人中被偶然诊断出。
鉴于其与多种遗传性疾病的关联、上尿路异常的发生频率以及潜在神经源性或梗阻性疾病的可能性,有人提出应对所有存在膀胱憩室的儿童进行染色体异常检测。42 对于单发先天性膀胱憩室患者,目前不推荐进行常规遗传学检测。44
表 6 膀胱憩室患儿就诊时的症状及相关发现
| 作者 | # 例数 | 尿路感染 | 下尿路症状 | 尿潴留 | 其他 | 膀胱输尿管返流 | 肾积水 |
|---|---|---|---|---|---|---|---|
| Alexander | 47 | 26 | 2 | 0 | 2 | 32 | 19 |
| Evangilidis | 21 | 19 | 12 | 0 | – | – | – |
| Marte | 16 | 15 | 9 | 0 | 6 | 7 | – |
| Bhat | 12 | 5 | – | 12 | 4 | 9 | 5 |
| Macedo | 10 | 5 | 3 | 1 | – | – | 4 |
| 合计 | 106 | 70 (66) | 26 (25) | 13 (12) | 12 (11) | 48 (45) | 28 (26) |
影像学评估
膀胱憩室常在膀胱超声检查中首先被发现,表现为源自膀胱后外侧壁的低回声囊性病灶。对影像学怀疑的憩室,其鉴别诊断包括脐尿管囊肿、外翻型输尿管囊肿、异位输尿管、腹股沟疝内的膀胱疝以及膀胱重复畸形。44 采用侧位和斜位投照的排尿性膀胱尿道造影是确立膀胱憩室诊断的金标准,并可同时评估膀胱输尿管返流(VUR).43 尤其在无 VUR 的病例中,可采用静脉肾盂造影或 CT 或 MR 尿路造影以明确憩室与输尿管之间的解剖关系。44 伴膀胱憩室的儿童应评估上尿路是否存在输尿管肾积水。
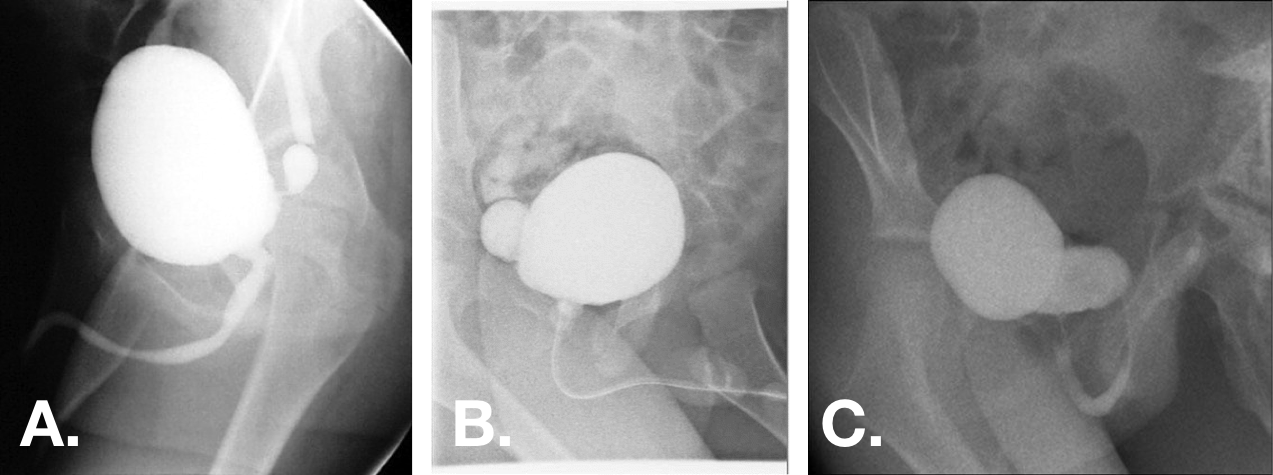
图 12 在 VCUG 斜位片上可见先天性膀胱憩室。憩室大小范围不等,可小至憩室颈极窄,亦可大于膀胱,且为宽基开口。44,47,55
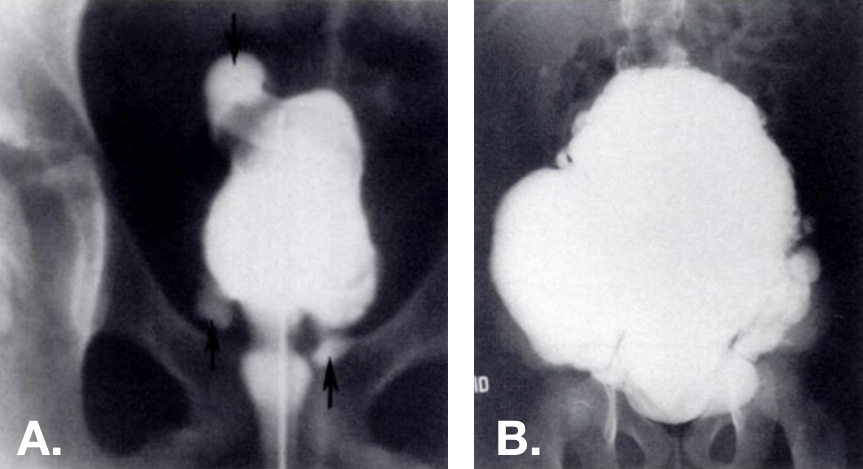
图 13 在 VCUG 上可见获得性憩室。多个憩室位于不同部位—包括膀胱穹隆部—,见于小梁化的膀胱。右侧的照片来自一名埃勒斯-当洛综合征患者。42
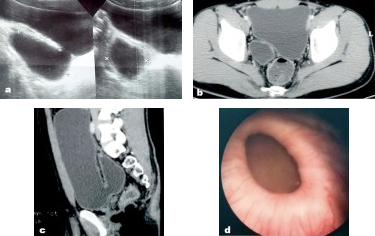
图 14 在超声、盆腔CT和膀胱镜检查中所见的先天性膀胱憩室。56
当怀疑存在潜在的下尿路功能障碍,或仅凭憩室的存在不足以充分解释患者的就诊症状时,可考虑对合并膀胱憩室的患者进行尿动力学检查。 约半数此类患者可见逼尿肌过度活动,可能继发于憩室内及其周围的低张力以及慢性尿液淤滞。44,48 憩室会使膀胱测压图的解读变得复杂,因为它们会降低膀胱内压。 憩室内的尿液淤滞及伴随的VUR也可能使结果复杂化。44
内镜评估可能有助于明确解剖结构,建议在手术干预过程中进行,但对于诊断膀胱憩室并非必需。
修复的适应证
尽管膀胱憩室在无干预的情况下不会自行消退,但并非所有患者都需要手术。对尿路感染(UTI)和排尿功能障碍进行充分治疗,可能缓解小而无并发症憩室患者的症状。44 对于症状难治或复杂性憩室者,手术切除通常可根治,且视需要可同时进行输尿管再植术。普遍接受的手术指征包括如下:43,44
- 对内科治疗难治的排尿症状
- 憩室 > 3cm
- 反复尿路感染
- 复杂性 VUR
- 尿潴留
- 上尿路梗阻或功能恶化
- 膀胱结石
- 憩室破裂
与成人不同,儿童膀胱憩室相关的上皮恶性转化通常不见;然而,一旦对恶性病变有任何担忧,则需要进行外科干预。43
在任何手术干预之前,应对排尿功能障碍进行评估和处理,以明确与憩室相关的症状及其术后演变。逼尿肌低活动、膀胱出口梗阻或脊髓裂畸形等相关病变,应在行憩室切除术之前予以处理。
对于存在潜在结缔组织疾病的患者,应谨慎考虑外科干预,因为该人群复发的可能性较高。44,53
手术途径
膀胱憩室切除术可经内镜实施,或通过经膀胱或膀胱外的开放或微创入路进行。经膀胱和膀胱外入路与输尿管再植术所用入路相同,如因憩室位置需要,或因伴随的严重VUR而有指征,可在手术中同时行再植术。建议在手术规划中经膀胱镜评估憩室开口与输尿管口的相对位置。多项病例系列报道,无论采用何种入路,憩室切除后症状可完全缓解—或排尿功能障碍症状显著减轻—以及VUR也得到解决。57,55 已有经输尿管口下方黏膜下注射填充剂以闭合输尿管旁憩室开口的报道,但关于其长期疗效的数据有限。58,59 一项对内镜、膀胱内和膀胱外入路的比较显示,VUR分别在79%、91%和80%的病例中成功解决。59
无论采用何种手术入路,重要的手术注意事项包括:由培养结果指导的围手术期抗菌预防,避免输尿管或输精管的损伤或血供破坏,修补大型后位憩室时避免直肠损伤,以及术后膀胱减压。44
要点
- 儿童的膀胱憩室可能为先天性—由于逼尿肌缺陷,最常见于Waldeyer鞘周围(Hutch 憩室)—或因神经源性或梗阻性下尿路病变而获得性。
- Hutch 憩室导致膀胱壁及输尿管肌性隧道变形,使儿童易发生膀胱输尿管返流。
- 儿童膀胱憩室最常表现为因憩室内尿潴留继发的尿路感染,或在针对肾积水或发热性尿路感染并怀疑膀胱输尿管返流的评估中被发现。大多数憩室无症状。
- 含侧位和斜位投照的排尿性膀胱尿道造影是确立膀胱憩室诊断的金标准,并可同时评估VUR。
- 对于症状难治或复杂性憩室者,可经膀胱入路或膀胱外入路行外科切除;必要时可同时行输尿管再植术。
- 在进行任何手术干预之前,应评估并处理排尿功能障碍。
摘要
脐尿管异常可有多种表现,最常见为脐部渗液或感染。部分异常可采取保守治疗,并可自行消退。感染性囊肿可经皮引流,任何有症状的异常均可通过开放或腹腔镜手术切除。儿童无症状脐尿管异常发生恶变的风险尚不明确。
前列腺小囊扩张与尿道下裂及其他泌尿生殖系统异常相关,可无症状,或导致与尿路出口梗阻和尿淤滞相关的症状。可通过 RUG、VCUG 或 US 诊断。对于有症状的患者,可通过内镜、开放或腹腔镜途径行切除术,其中内镜治疗的复发风险更高。
儿科膀胱憩室可为先天性或后天性。先天性憩室是由于逼尿肌在输尿管鞘附近的缺损所致,并与膀胱输尿管返流(VUR)以及结缔组织病有关。憩室常无症状,但可导致感染、刺激性排尿症状、尿潴留或腹痛,并常与逼尿肌过度活动相关。后天性憩室多继发于下尿路梗阻或神经源性膀胱。手术修补的适应证包括难治性或严重症状、大小 >3厘米,以及合并的泌尿道功能障碍。在干预前应先处理其他任何下尿路病变。关于手术方式尚无共识,且常同时行输尿管再植术。
参考文献
- Jm P. Embryology of the genitourinary tract. Elsevier: Philadelphia; 2016, DOI: 10.1201/b13795-15.
- MacNeily AE, Koleilat N, Kiruluta HG, Homsy YL. Urachal abscesses: Protean manifestations, their recognition, and management. Urology 1992; 40 (6): 530–535. DOI: 10.1016/0090-4295(92)90409-p.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg 2013; 48 (10): 2148–2152. DOI: 10.1016/j.jpedsurg.2013.02.069.
- Fox JA, M.S. R, JC G, CF A, RA H, JC V, et al.. Vesicoureteral reflux in children with urachal anomalies. J Pediatr Urol 2011; 7 (6): 632–635. DOI: 10.1016/j.jpurol.2011.04.001.
- Ashley RA, Inman BA, Routh JC, Rohlinger AL, Husmann DA, Kramer SA. Urachal Anomalies: A Longitudinal Study of Urachal Remnants in Children and Adults. J Urol 2007; 178 (4s): 1615–1618. DOI: 10.1016/j.juro.2007.03.194.
- Cilento BG, Bauer SB, Retik AB, Peters CA, Atala A. Urachal anomalies: defining the best diagnostic modality. Urology 1998; 52 (1): 120–122. DOI: 10.1016/s0090-4295(98)00161-7.
- Rich RH, Hardy BE, Filler RM. Surgery for Anomalies of the Urachus. J Urol 1983; 131 (3): 616–616. DOI: 10.1016/s0022-5347(17)50535-2.
- Yiee JH, Garcia N, Baker LA, Barber R, Snodgrass WT, Wilcox DT. A diagnostic algorithm for urachal anomalies. J Pediatr Urol 2007; 3 (6): 500–504. DOI: 10.1016/j.jpurol.2007.07.010.
- Copp HL, Wong IY, Krishnan C, Malhotra S, Kennedy WA. Clinical Presentation and Urachal Remnant Pathology: Implications for Treatment. J Urol 2009; 182 (4s): 1921–1924. DOI: 10.1016/j.juro.2009.03.026.
- Cuda SP, Vanasupa BP, Sutherland RS. Nonoperative management of a patent urachus. Urology 2005; 66 (6): 1320.e7–1320.e9. DOI: 10.1016/j.urology.2005.06.121.
- Gleason JM, Bowlin PR, Bagli DJ, Lorenzo AJ, Hassouna T, Koyle MA, et al.. A Comprehensive Review of Pediatric Urachal Anomalies and Predictive Analysis for Adult Urachal Adenocarcinoma. J Urol 2015; 193 (2): 632–636. DOI: 10.1016/j.juro.2014.09.004.
- Stopak JK, Azarow KS, Abdessalam SF, Raynor SC, Perry DA, Cusick RA. Trends in surgical management of urachal anomalies. J Pediatr Surg 2015; 50 (8): 1334–1337. DOI: 10.1016/j.jpedsurg.2015.04.020.
- Dethlefs CR, Abdessalam SF, Raynor SC, Perry DA, Allbery SM, Lyden ER, et al.. Conservative management of urachal anomalies. J Pediatr Surg 2019; 54 (5): 1054–1058. DOI: 10.1016/j.jpedsurg.2019.01.039.
- Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant Urachal Lesions. J Urol 1984; 131 (1): 1–8. DOI: 10.1016/s0022-5347(17)50167-6.
- Oestreich AE. Urachal anomalies in children: the vanishing relevance of the preoperative voiding cystourethrogram. Yearbook of Diagnostic Radiology 2005; 2007 (12): 174–175. DOI: 10.1016/s0098-1672(08)70123-1.
- Lewis JB, Morse JW, Eyolfson MF, Schwartz SL. Spontaneous Rupture of a Vesicourachal Diverticulum Manifesting as Acute Abdominal Pain. Acad Emerg Med 1996; 3 (12): 1140–1143. DOI: 10.1111/j.1553-2712.1996.tb03375.x.
- OGBEVOEN JUSTINO, JAFFE DAVIDM, LANGER JACOBC. Intraperitoneal rupture of an infected urachal cyst: A rare cause of peritonitis in children. Pediatr Emerg Care 1996; 12 (1): 41–43. DOI: 10.1097/00006565-199602000-00012.
- GONZ??LEZ RICARDO, De FILIPPO ROGER, JEDNAK ROMAN, BARTHOLD JULIASPENCER. Urethral Atresia: Long-term Outcome In 6 Children Who Survived The Neonatal Period. J Urol 2001: 2241–2244. DOI: 10.1097/00005392-200106001-00006.
- Mesrobian H-GO, Zacharias A, Balcom AH, Cohen RD. Ten Years of Experience With Isolated Urachal Anomalies in Children. J Urol 1997; 158: 1316–1318. DOI: 10.1097/00005392-199709000-00173.
- McCollum MO, MacNeily AE, Blair GK. Surgical implications of urachal remnants: Presentation and management. J Pediatr Surg 2003; 38 (5): 798–803. DOI: 10.1016/jpsu.2003.50170.
- Minevich E, W.J. L, A.G.. The infected urachal cyst: Primary excision versus a staged approach. J Pediatr Surg 1997; 33 (1): 147. DOI: 10.1016/s0022-3468(98)90408-0.
- Newman BM, Karp MP, Jewett TC, Cooney DR. Advances in the management of infected urachal cysts. J Pediatr Surg 1986; 21 (12): 1051–1054. DOI: 10.1016/0022-3468(86)90006-0.
- Yoo KH, Lee S-J, Chang S-G. Treatment of Infected Urachal Cysts. Yonsei Med J 2006; 47 (3): 423. DOI: 10.3349/ymj.2006.47.3.423.
- Galati VG, D.B. R, F.. Management of Urachal Remnants in Early Childhood. Yearbook of Diagnostic Radiology 2008; 2009: 154–155. DOI: 10.1016/s0098-1672(09)79312-9.
- Nogueras-Ocaña M, Rodríguez-Belmonte R, Uberos-Fernández J, Jiménez-Pacheco A, Merino-Salas S, Zuluaga-Gómez A. Urachal anomalies in children: Surgical or conservative treatment? J Pediatr Urol 2014; 10 (3): 522–526. DOI: 10.1016/j.jpurol.2013.11.010.
- Lipskar AM, Glick RD, Rosen NG, Layliev J, Hong AR, Dolgin SE, et al.. Nonoperative management of symptomatic urachal anomalies. J Pediatr Surg 2010; 45 (5): 1016–1019. DOI: 10.1016/j.jpedsurg.2010.02.031.
- Stone NM, Garden RJ, Webert H. Laparoscopic excision of a urachal cyst. Urology 1995; 45 (1): 161–164. DOI: 10.1016/s0090-4295(95)97824-0.
- OKEGAWA TAKATSUGU, ODAGANE AKIHIRO, NUTAHARA KIKUO, HIGASHIHARA EIJI. Laparoscopic management of urachal remnants in adulthood. Int J Urol 2005; 13 (12): 1466–1469. DOI: 10.1111/j.1442-2042.2006.01613.x.
- Yamzon J, K.P. DF, RE C, AY H, BE K, C.J.. V1710 Pediatric Robotic-assisted Laparoscopic Urachal Cyst And Bladder Cuff Excision. J Urol 2008; 185 (4s): 2385–2388. DOI: 10.1016/j.juro.2011.02.2035.
- Patrzyk M, Glitsch A, Schreiber A, Bernstorff W von, Heidecke C-D. Single-incision laparoscopic surgery as an option for the laparoscopic resection of an urachal fistula: first description of the surgical technique. Surg Endosc 2010; 24 (9): 2339–2342. DOI: 10.1007/s00464-010-0922-4.
- IKOMA F, SHIMA H, YABUMOTO H. Classification of Enlarged Prostatic Utricle in Patients with Hypospadias. Br J Urol 1985; 57 (3): 334–337. DOI: 10.1111/j.1464-410x.1985.tb06356.x.
- Aktuğ T, Ekberli\. G. Re: The prostatic utricle: an under-recognized condition resulting in significant morbidity in boys with both hypospadias and normal external genitalia. J Pediatr Urol 2017; 15 (4): 425. DOI: 10.1016/j.jpurol.2019.03.030.
- Dai L-N, He R, Wu S-F, Zhao H-T, Sun J. Surgical treatment for prostatic utricle cyst in children: A single-center report of 15 patients. Int J Urol 2021; 28 (6): 689–694. DOI: 10.1111/iju.14543.
- Devine CJ, Gonzalez-Serva L, Stecker JF, Devine PC, Horton CE. Utricular Configuration in Hypospadias and Intersex. J Urol 1980; 123 (3): 407–411. DOI: 10.1016/s0022-5347(17)55959-5.
- Liu B, He D, Zhang D, Liu X, Lin T, Wei G. Prostatic utricles without external genital anomalies in children: our experience, literature review, and pooling analysis. BMC Urol 2019; 19 (1): 21. DOI: 10.1186/s12894-019-0450-z.
- Farikullah J, Ehtisham S, Nappo S, Patel L, Hennayake S. Persistent Müllerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Müllerian remnants. BJU Int 2012; 110 (11c): E1084–e1089. DOI: 10.1111/j.1464-410x.2012.11184.x.
- Desautel MG, Stock J, Hanna MK, REMNANTS MULLERIANDUCT. Müllerian Duct Remnants. J Urol 1999; 162 (3, Part 2): 1014. DOI: 10.1097/00005392-199909000-00013.
- Jia W, L.G. Z, L W, Y F, W H, J X, et al.. Comparison of laparoscopic excision versus open transvesical excision for symptomatic prostatic utricle in children J Pediatr Surg. 2016; 51 (10): 1597–1601. DOI: 10.1016/j.jpedsurg.2016.06.004.
- Babu R, Chandrasekharam VVS. Cystoscopic Management of Prostatic Utricles. Urology 2021; 149: e52–e55. DOI: 10.1016/j.urology.2020.09.005.
- Nallabothula AK, Yatam LS, Ayapati T, Bodagala VD. Large Prostatic Utricle With Transitional Cell Carcinoma in an Adult. Urology 2017; 102: e5–e6. DOI: 10.1016/j.urology.2016.12.048.
- Yeung CK, Sihoe JDY, Tam YH, Lee KH. Laparoscopic excision of prostatic utricles in children. BJU Int 2001; 87 (6): 505–508. DOI: 10.1046/j.1464-410x.2001.00132.x.
- Johnson D, Parikh K, Schey W, Mar W. MRI in Diagnosis of a Giant Prostatic Utricle. Case Rep Radiol 2014; 2014: 1–3. DOI: 10.1155/2014/217563.
- Kojima Y, Hayashi Y, Maruyama T, Sasaki S, Kohri K. Comparison between ultrasonography and retrograde urethrography for detection of prostatic utricle associated with hypospadias. Urology 2001; 57 (6): 1151–1155. DOI: 10.1016/s0090-4295(01)00954-2.
- Bayne AP, Austin JC, Seideman CA. Robotic assisted retrovesical approach to prostatic utricle excision and other complex pelvic pathology in children is safe and feasible. J Pediatr Urol 2021; 17 (5): 710–715. DOI: 10.1016/j.jpurol.2021.08.004.
- Blane CE, Zerin JM, Bloom DA. Bladder diverticula in children. Radiology 1994; 190 (3): 695–697. DOI: 10.1148/radiology.190.3.8115613.
- Es R. Bladder and female urethral diverticula. Elsevier: Philadelphia; 2017, DOI: 10.1016/b978-1-4160-6911-9.00078-5.
- Psutka SP, Cendron M. Bladder diverticula in children. J Pediatr Urol 2013; 9 (2): 129–138. DOI: 10.1016/j.jpurol.2012.02.013.
- Sammour Z, Gomes C, Bessa J, Pinheiro M, Kim C, Sacomani C, et al.. 1622 Congenital Genitourinary Abnormalities In Children With Williams-beuren Syndrome. J Urol 2014; 187 (4s): 804–809. DOI: 10.1016/j.juro.2012.02.1417.
- Bhat A, Bothra R, Bhat MP, Chaudhary GR, Saran RK, Saxena G. Congenital bladder diverticulum presenting as bladder outlet obstruction in infants and children. J Pediatr Urol 2012; 8 (4): 348–353. DOI: 10.1016/j.jpurol.2011.07.001.
- Alexander RE, Kum JB, Idrees M. Bladder Diverticulum: Clinicopathologic Spectrum in Pediatric Patients. Pediatr Dev Pathol 2012; 15 (4): 281–285. DOI: 10.2350/12-02-1154-oa.1.
- Evangelidis A, Castle EP, Ostlie DJ, Snyder CL, Gatti JM, Murphy JP. Surgical management of primary bladder diverticula in children. J Pediatr Surg 2005; 40 (4): 701–703. DOI: 10.1016/j.jpedsurg.2005.01.003.
- Marte A, Cavaiuolo S, Esposito M, Pintozzi L. Vesicoscopic Treatment of Symptomatic Congenital Bladder Diverticula in Children: A 7-Year Experience. Eur J Pediatr Surg 2015; 26 (03): 240–244. DOI: 10.1055/s-0035-1551564.
- Cruz ML da, Macedo A, Garrone G, Ottoni SL, Oliveira DE, Rosário Souza GRM do. Primary congenital bladder diverticula: Where does the ureter drain? Afr J Paediatr Surg 2015; 12 (4): 280. DOI: 10.4103/0189-6725.172574.
- Livne PM, Gonzales ET. Congenital bladder diverticula causing ureteral obstruction. Urology 1985; 25 (3): 273–276. DOI: 10.1016/0090-4295(85)90327-9.
- Stein RJ, Matoka DJ, Noh PH, Docimo SG. Spontaneous perforation of congenital bladder diverticulum. Urology 2005; 66 (4): 881.e5–881.e6. DOI: 10.1016/j.urology.2005.04.004.
- Britto MM, Yao HHI, Campbell N. Delayed diagnosis of spontaneous rupture of a congenital bladder diverticulum as a rare cause of an acute abdomen. ANZ J Surg 2019; 89 (9): 385–387. DOI: 10.1111/ans.14559.
- Jorion JL, Michel M. Spontaneous rupture of bladder diverticula in a girl with Ehlers-Danlos syndrome. J Pediatr Surg 1999; 34 (3): 483–484. DOI: 10.1016/s0022-3468(99)90506-7.
- Temiz A, Akcora B, Atik E. An atypical bladder diverticulum presented with recurrent peritonitis: case report. Ulus Travma Acil Cerrahi Derg 2011; 17 (4): 365–367. DOI: 10.5505/tjtes.2011.81542.
- Christman MS, Casale P. Robot-Assisted Bladder Diverticulectomy in the Pediatric Population. J Endourol 2012; 26 (10): 1296–1300. DOI: 10.1089/end.2012.0051.
- Nerli RB, Ghagane SC, Musale A, Deole S, Hiremath MB, Dixit NS, et al.. Congenital bladder diverticulum in a child: Surgical steps of extra-vesical excision. Urol Case Rep 2019; 22: 42–43. DOI: 10.1016/j.eucr.2018.10.009.
- Cerwinka WH, Scherz HC, Kirsch AJ. Endoscopic Treatment of Vesicoureteral Reflux with Dextranomer/Hyaluronic Acid in Children. Adv Urol 2008; 2008: 1–7. DOI: 10.1155/2008/513854.
- Aydogdu O, Burgu B, Soygur T. Predictors of Surgical Outcome in Children With Vesicoureteral Reflux Associated With Paraureteral Diverticula. Urology 2010; 76 (1): 209–214. DOI: 10.1016/j.urology.2009.11.052.
最近更新时间: 2025-09-22 08:00