26: Estenose uretral congênita e traumática
Este capítulo levará aproximadamente 13 minutos para ler.
Introdução
As estenoses uretrais foram inicialmente consideradas incomuns em crianças.1 Relatos posteriores sugeriram que elas não são tão raras.2 A impressão de baixa incidência devia-se principalmente à subnotificação e à escassez de literatura. Johanson foi o primeiro a observar a formação de estenose em 1953 após ruptura uretral completa.1 As estenoses uretrais são amplamente classificadas com base na sua etiologia em congênitas/idiopáticas, iatrogênicas, inflamatórias e traumáticas. Não está claro se as estenoses sem causa definida devem ser classificadas como congênitas ou idiopáticas.
Harshman et al e Kaplan & Brock recomendaram evitar a designação de estenose uretral congênita e descreveram tais lesões como estenoses de etiologia desconhecida.3,4 Não está claro se as estenoses induzidas por cateter podem ser classificadas como iatrogênicas ou inflamatórias. Uma vez que tal estenose não teria ocorrido sem um cateter de demora em primeiro lugar, pode-se argumentar que ela deveria ser classificada como iatrogênica.2 Causas iatrogênicas, como cateterização ou estenose após correção de hipospádia, respondem pela maioria dos casos de estenose uretral anterior na população pediátrica, especialmente na faixa etária mais jovem. Entretanto, à medida que a criança cresce, há uma preponderância gradual de estenoses uretrais traumáticas, incluindo estenoses uretrais posteriores.
Estenoses Congênitas e Idiopáticas
Mori et al relataram que a estenose/estreitamento uretral congênito era uma causa importante de infecções do trato urinário recorrentes, enurese, aumento da frequência urinária ou hematúria em meninos.5 É reconhecida como um preenchimento linear típico (Figura 1) Também chamada de colar de Cobb ou anel de Moorman ou membrana uretral posterior obstrutiva congênita (COPUM), sua etiologia é obscura. Debate-se se essa entidade poderia ser denominada uma estenose.
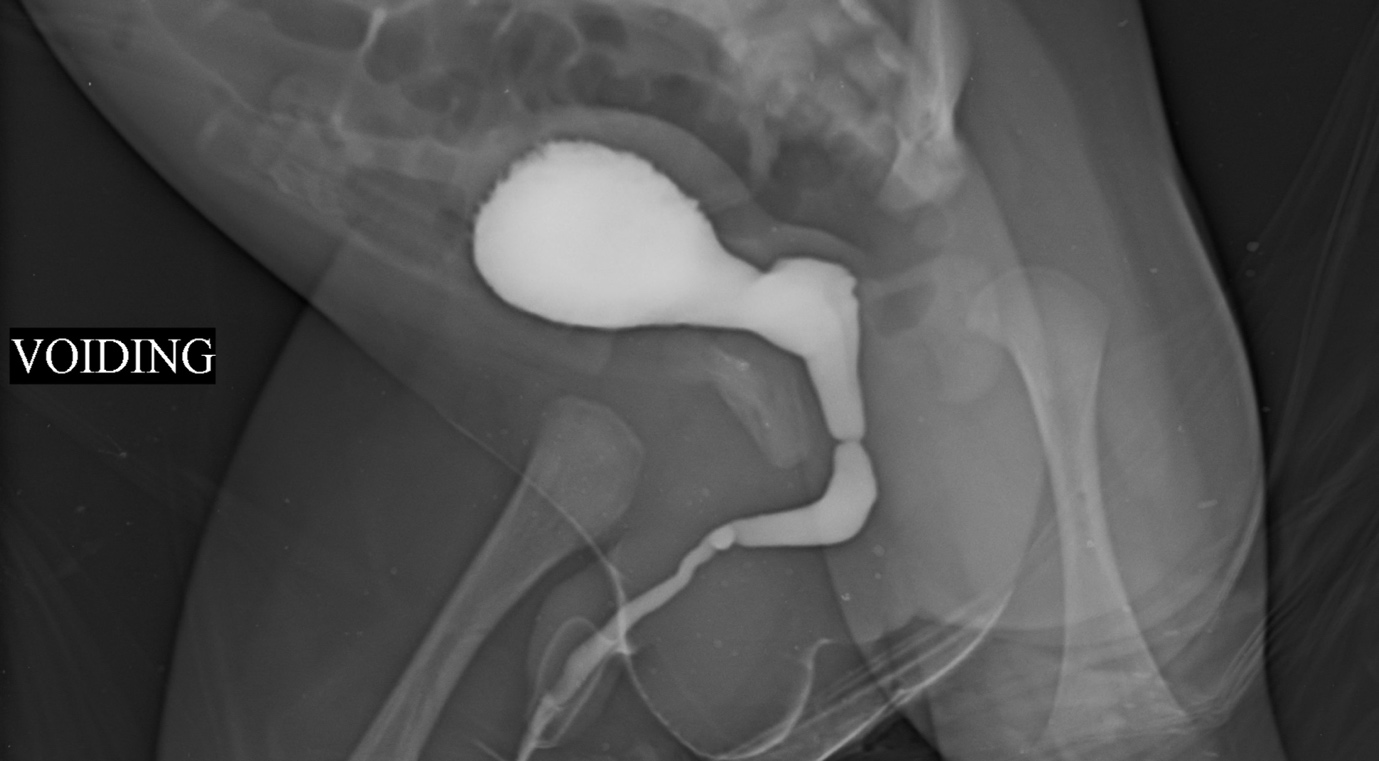
Figura 1 VCUG mostrando estenose/ estreitamento uretral idiopática/ congênita. Estreitamento em anel é observado na junção da uretra anterior com a uretra posterior. Frequentemente, as vias urinárias superiores não estão dilatadas e os pacientes se apresentam com ITU ou gotejamento de urina
Essa lesão é observada como uma estenose em forma de anel na cistoscopia (Figura 2) logo distal ao esfíncter uretral externo.6 Tipicamente, características como irregularidade vesical, espessamento ou alterações do trato urinário superior vistas nas válvulas uretrais posteriores (PUV) estão ausentes. O tratamento mais eficaz dessa lesão é a uretrotomia interna óptica (OIU) sob visão direta. Este procedimento é realizado na posição de 12 horas utilizando uma lâmina fria (seta de (Figura 2) Em meninos com incontinência urinária diurna e estenose uretral bulbar congênita, os episódios de incontinência melhoraram em 69.4% após a OIU.5 Além disso, infecções do trato urinário e refluxo vesicoureteral foram reduzidos com o tratamento imediato dessa patologia.
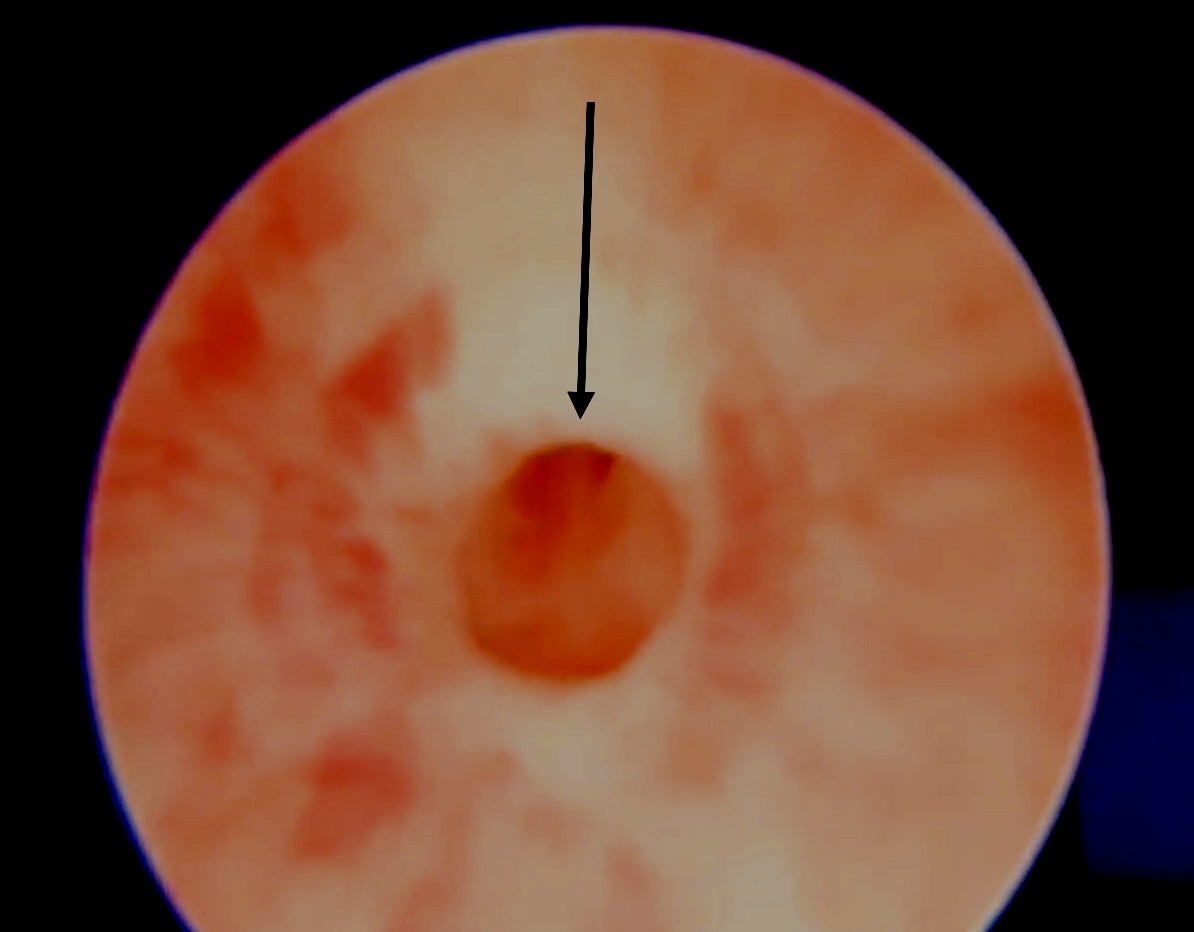
Figura 2 Uretrotomia interna ótica (OIU) para estreitamento idiopático/ congênito envolve seccionamento com lâmina fria na posição das 12 horas
Estenoses iatrogênicas
Estenoses induzidas por cateter
A cateterização uretral é uma importante causa iatrogênica (12.3% no total) e, em um estudo de Ansari et al., é a principal causa de estenose uretral multifocal e pan-anterior.2 Estenoses iatrogênicas decorrentes de cateterização prolongada (muitas vezes inserida para monitorização do débito em UTIs neurológicas) localizam-se frequentemente na região bulbomembranosa. Embora algumas possam dever-se a cateteres superdimensionados, outras podem resultar da insuflação do balão na uretra ou da remoção traumática do cateter (nessa situação, sangramento uretral é frequentemente observado). A própria cateterização prolongada provoca inflamação uretral, isquemia e, por fim, estenose uretral. Frequentemente, apresentam-se com retenção após a retirada do cateter. Nessa situação, o autor prefere realizar a inserção de um cateter suprapúbico (SPC) sob orientação por ultrassom. Isso auxilia em um futuro teste de micção por meio do clampeamento do SPC. O SPC também pode ser utilizado para uma cistouretrografia miccional (VCUG) a fim de avaliar a extensão da estenose. Muitas vezes, essas estenoses são estreitamentos de segmento curto, passíveis de OIU sob anestesia geral. Após a OIU, são necessários um novo VCUG e um teste de micção para garantir a resolução da estenose. Indicações claras e bem definidas para cateterização, inserção habilidosa do cateter uretral e consideração de SPC quando for provável cateterização prolongada devem reduzir a incidência dessas estenoses iatrogênicas.
Estenoses após válvula uretral posterior
A fulguração transuretral de válvulas é outra causa importante de estenose uretral pediátrica. Embora faltem dados específicos sobre a contribuição da fulguração de válvula para estenoses pediátricas, a incidência varia de 0% a 25% em diversas séries.7 Em uma série mais recente, 11 de 62 pacientes (5,6% do total) apresentaram estenose iatrogênica após fulguração de válvula.2 Embora várias causas tenham sido propostas no desenvolvimento dessas estenoses, a mais importante é a inserção traumática de um ressectoscópio de calibre excessivo em um lúmen uretral estreito e a fuga de corrente monopolar devido a isolamento insuficiente do ressectoscópio ou uso excessivo do eletrocautério. Considera-se que esta é uma causa evitável de estenoses em crianças, que pode ser prevenida pela delicadeza na técnica cirúrgica, uso de instrumentos pequenos de calibre apropriado para fulguração numa uretra infantil estreita, redução do tempo de contato durante a fulguração para evitar cortes profundos e fulguração sob visão direta apenas na área das válvulas.8 Os autores preferem utilizar ablação com lâmina fria em vez de fulguração por diatermia para a ablação de PUV a fim de prevenir lesão térmica e reduzir a formação de estenose.9 Frequentemente, as estenoses após ablação de válvula são estenoses de segmento curto, passíveis de UIO. Este procedimento deve ser realizado cuidadosamente, com passagem prévia de um fio-guia.
Estenoses uretrais na hipospádia
Estenoses após reparo de hipospádia representam uma boa proporção das estenoses uretrais anteriores em adolescentes e adultos jovens. Podem apresentar-se precocemente com jato fraco e gotejamento ou tardiamente com infecções do trato urinário (ITU) recorrentes e alterações do trato urinário superior. A apresentação é altamente variável, e podem ser diagnosticadas tardiamente, especialmente no início da vida adulta após um reparo supostamente ‘bem-sucedido’ na infância sem fístulas. Ansari enfatizou a necessidade de alertar os pais de que crianças submetidas a reparo de hipospádia podem desenvolver estenoses no futuro e devem ser monitoradas durante a adolescência quanto à formação de estenose.2 Frequentemente, a urofluxometria mostra um traçado plano com grande resíduo urinário pós-miccional. Estenoses de hipospádia após reparo uretral distal (placa incisada tubularizada- TIP) são frequentemente estenoses de segmento longo devido a lúmen uretral deficiente. Apresentam-se precocemente com esforço miccional e gotejamento. Frequentemente precisam ser abertas até que se atinja a uretra de calibre normal. Podem necessitar de enxerto cutâneo prepucial local/ enxerto de mucosa oral (OMG) ou enxerto de mucosa bucal (BMG) como inlay enquanto são abertas. Após um período de 6 meses, podem ser tubularizadas para fornecer uma uretra de calibre mais amplo. Estenose de hipospádia após reparo da uretra proximal (após um reparo tubularizado em tempo único – Duckett) frequentemente se deve a estreitamento na junção da uretra nativa com a neouretra revestida por pele. Uma uretrografia ascendente (AUG) é necessária para demonstrar a localização exata e a extensão do estreitamento (Figura 3) Se for encontrada uma estenose em anel estreito na junção da neouretra com a uretra nativa, uma OIU isolada pode ser suficiente. Por outro lado, uma estenose densa de segmento longo pode necessitar de reparo em estágios com OMG/BMG.10 (Figura 4) representa um fluxograma no manejo das estenoses uretrais anteriores de hipospádia (HAUS). Todo o segmento estreito e cicatricial é excisado e o OMG é fixado com quilting no local. Após um período de 6 meses a 1 ano, realiza-se a tubularização. Barbagli relatou que o comprimento da estenose, mas não o número de operações prévias necessárias para o reparo primário da hipospádia, esteve associado ao risco de falha.11 Alguns cirurgiões preferem um reparo dorsal com OMG em inlay em tempo único para essas situações, enquanto o autor prefere uma abordagem em dois tempos.12,13
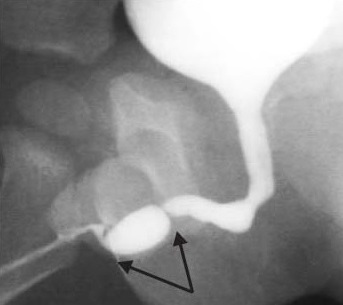
Figura 3 Estenose uretral anterior após correção de hipospádia frequentemente observada na junção entre a uretra nativa e a neouretra. Estreitamentos múltiplos em pregas são passíveis de OIU, enquanto estenoses uretrais anteriores longas requerem enxerto de mucosa oral com reparo em duas etapas.
Figura 4 Um fluxograma para o manejo de estenoses uretrais anteriores associadas à hipospádia (HAUS).
Estenoses Inflamatórias
Ao contrário das séries em adultos, estenoses decorrentes de líquen escleroso (LE) ou de causas infecciosas são raras em crianças.14 Embora o LE seja frequentemente considerado uma doença da vida adulta, a balanite xerótica obliterante (BXO), semelhante ao LE, é uma causa importante de estenoses uretrais, especialmente em crianças mais velhas. Relatos recentes mostram que não são tão incomuns quanto se pensava.15 Essas estenoses são mais difíceis de tratar e exigem múltiplas intervenções, pois representam um processo inflamatório crônico e têm tendência à recorrência.2 Urologistas pediátricos que tratam essas estenoses devem explicar aos cuidadores sobre a condição e a necessidade de acompanhamento prolongado para identificar recorrências.
Uretroplastia de substituição com enxerto de mucosa bucal e oral
Estenoses uretrais anteriores que não são passíveis de anastomose término-terminal exigem uretroplastia de substituição. Isto pode ser alcançado usando retalhos de pele peniana ou enxertos livres de pele de espessura total, mucosa oral da bexiga (OMG) ou mucosa bucal (BMG). A uretroplastia de substituição com OMG/ BMG vem emergindo rapidamente como a técnica mais preferível para estenoses panuretrais envolvendo a uretra distal.16 Naqueles com estenoses da uretra pendular utiliza-se uma incisão circum-coronal, enquanto para estenoses mais proximais se usa uma incisão perineal mediana. O esponjoso é então descolado dorsalmente dos corpos e realiza-se uma uretrotomia na posição das 12 horas. Em pacientes com estenoses inflamatórias ou traumáticas, essa abordagem é mais fácil, enquanto naqueles com estenose por hipospádia o esponjoso está tipicamente cicatrizado/ausente, tornando viável apenas a abertura ventray lay na posição das 6 horas. Nesses casos, um procedimento em dois tempos envolve a excisão completa do tecido fibrótico e BMG quilting na primeira etapa. A uretra é reconstruída na segunda etapa, 4–6 meses após a primeira.
Em um reparo em um único estágio com onlay dorsal, para estenoses inflamatórias ou traumáticas, o BMG/OMG é fixado aos corpos cavernosos com suturas interrompidas de poliglactina 6–0 e, subsequentemente, suturado às margens seccionadas da uretra com suturas contínuas. Barbagli et al descreveram as etapas operatórias detalhadas (Figura 5)17 Para a reconstrução meatal, o BMG mais distal é fixado com suturas interrompidas de poliglactina 5–0 às margens do meato cortadas dorsalmente. O paciente recebe alta 3–5 dias após a cirurgia com um cateter uretral de demora e um cateter suprapúbico. Os cateteres são removidos 3 semanas depois, após uma cistouretrografia miccional. Embora alguns autores favoreçam a técnica de onlay dorsal, outros relataram excelentes resultados a longo prazo com técnicas de onlay ventral,5,18,19
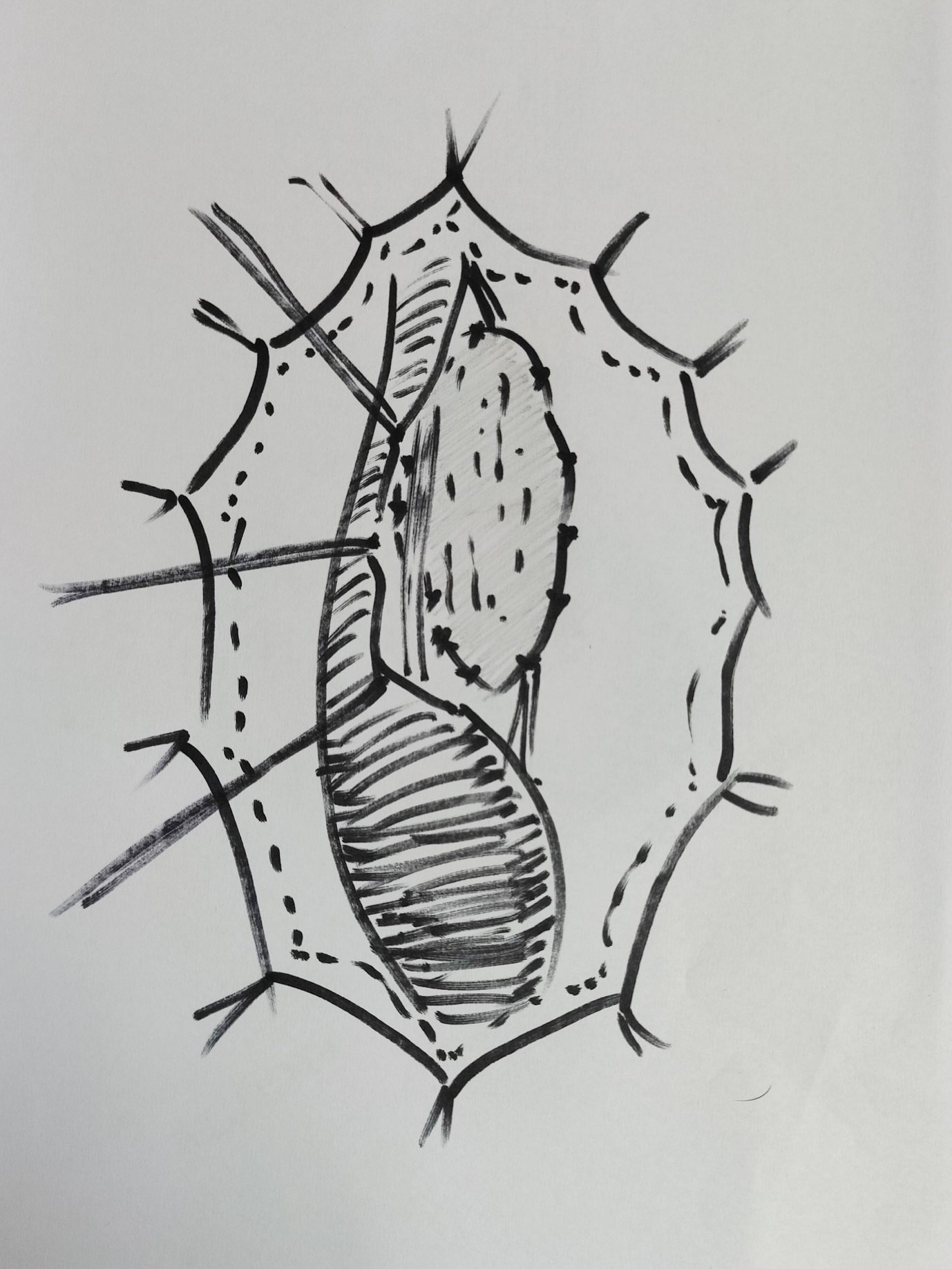
Figura 5 Uretroplastia de substituição com onlay dorsal de BMG; a uretra foi mobilizada; aberta na posição das 12 horas dorsalmente; BMG acolchoado dorsalmente e sendo anastomosado às bordas.
Estenoses traumáticas
A etiologia traumática torna-se um importante contribuinte para a doença por estenose uretral à medida que a idade avança. Em uma série de Ansari, 36,9% das estenoses no geral foram de etiologia traumática.2 Enquanto 18% das estenoses em crianças com menos de 10 anos eram secundárias a trauma, 45% das estenoses em pacientes com mais de 10 anos eram de origem traumática. A fratura pélvica, uma causa importante de estenose uretral em adolescentes, geralmente ocorre após queda de altura, acidentes de trânsito ou trauma perineal por cavalgamento.
Turner Warwick introduziu o termo “complexo” defeito de distração uretral posterior por fratura pélvica (PFPUDD) quando uma ou mais das seguintes características estão presentes: (a) o comprimento do defeito de distração é longo (≥3 cm), rodeado por fibrose pélvica extensa, e (b) são acompanhados por divertículos parauretrais, trajetos falsos ou lesão simultânea do colo vesical.20 A maior parte dos defeitos complexos de distração uretral requer uma exposição cirúrgica mais ampla para restaurar a continuidade uretral.21
Embora a patogênese de PFPUDD em crianças tenda a seguir um padrão semelhante ao dos adultos, vários elementos-chave requerem consideração. A localização da lesão uretral traumática em crianças é frequentemente imprevisível devido à posição abdominal da bexiga e à imaturidade da próstata.22 Outros fatores a serem considerados em crianças são: (a) os defeitos de distração uretral tendem a ser mais longos do que nos adultos devido ao marcado deslocamento cranial da bexiga e da próstata, (b) lesões duplas no colo vesical e na uretra membranosa são observadas com mais frequência em crianças e (c) o tamanho pré-puberal do períneo pode dificultar o alcance de uma extremidade uretral proximal situada alta.23
Colapinto e McCallum24 classificaram as lesões uretrais posteriores traumáticas em três categorias com base no aspecto radiológico. No tipo 1 a próstata ou o diafragma urogenital está deslocado mas a uretra membranosa está apenas distendida e não seccionada. No tipo 2 a uretra membranosa está rompida acima do diafragma urogenital no ápice da próstata. No tipo 3 a uretra membranosa está rompida acima e abaixo do diafragma urogenital Recentemente, uma nova classificação da lesão uretral posterior em pacientes com pelve fraturada foi proposta.25 O novo esquema de classificação nos permite comparar diferentes estratégias terapêuticas e seus desfechos (Tabela 1)
Tabela 1 Classificação anatómica e mecânica unificada das estenoses uretrais traumáticas.
| Classe | Definição |
|---|---|
| I | A uretra posterior distendida, porém íntegra |
| II | Ruptura da uretra prostato-membranosa acima do diafragma urogenital |
| III | Ruptura parcial ou completa de ambas as uretras, anterior e posterior, com disrupção do diafragma urogenital |
| IV | Lesão vesical com extensão para a uretra |
| IVa | Lesão da base vesical com extravasamento periuretral, simulando lesão da uretra posterior |
| V | Lesão pura da uretra anterior, parcial ou completa |
Ainda há debate entre urologistas pediátricos quanto à abordagem superior: realinhamento uretral precoce com ou sem reconstrução primária da uretra seccionada vs. SPC primária e reparo tardio da uretra. Nerli et al.26 relataram que metade das crianças submetidas ao realinhamento primário necessitou de uretrotomias endoscópicas adicionais, enquanto algumas necessitaram de uretroplastia para tratar uma estenose resultante. O autor prefere SPC primária para superar a fase aguda de retenção após o trauma. Também preferimos evitar a instrumentação uretral que possa causar dano uretral adicional ou uma exploração pélvica arriscada que pode perturbar um hematoma e impedir uma aproximação adequada. Após um período de 6-8 meses, quando a estabilização ortopédica é removida, realiza-se uma uretrografia em oposição para avaliar a localização e o comprimento da estenose (Figura 6) Vários procedimentos cirúrgicos foram propostos para o reparo tardio dos PFPUDDs. Estes incluem dilatação uretral, técnicas endoscópicas como OIU, procedimentos de substituição e reparo anastomótico tardio sem tensão.
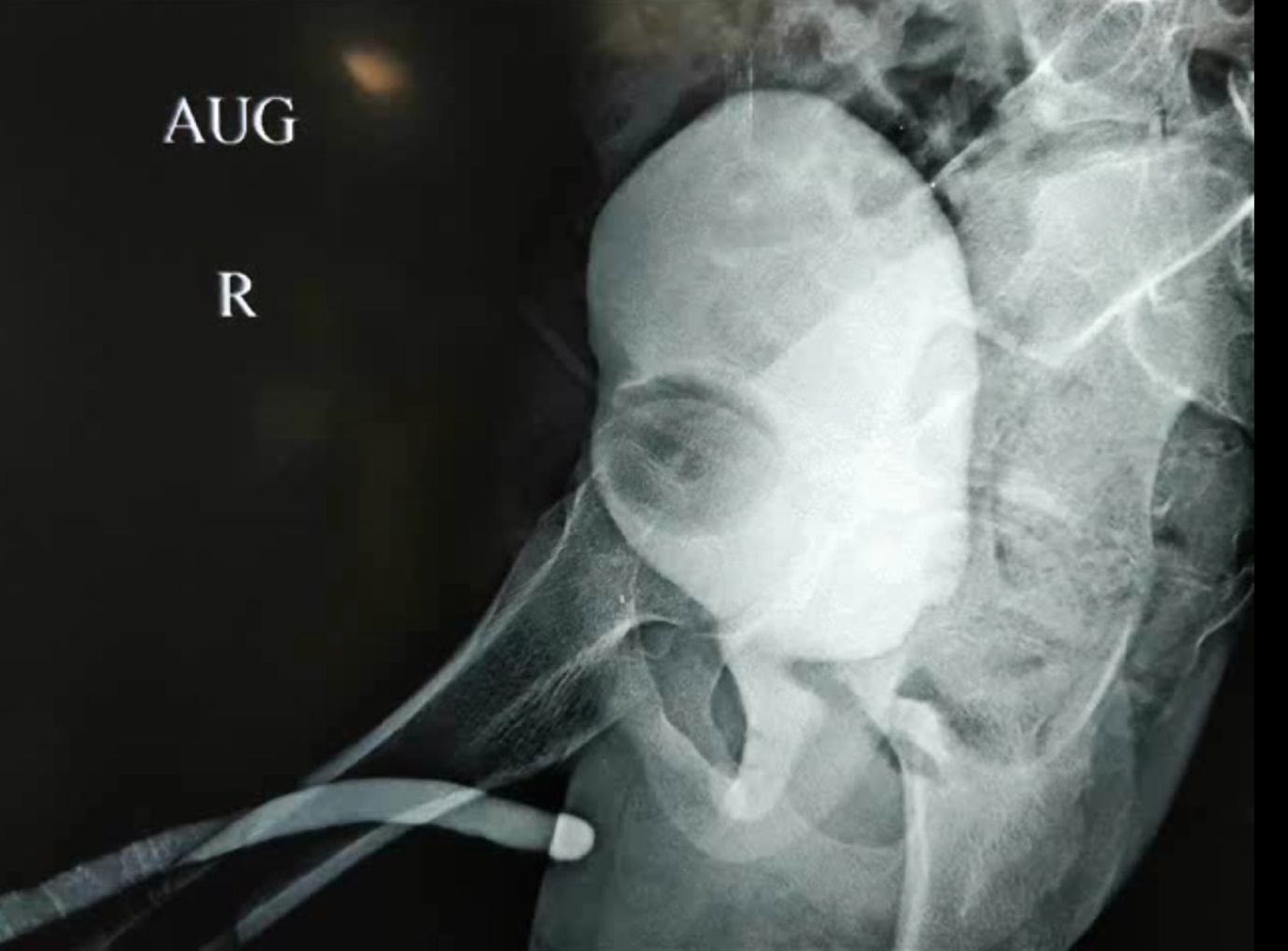
Figura 6 A uretrografia em oposição é uma etapa essencial na avaliação da extensão dos defeitos de distração uretral pós-traumáticos. É tipicamente realizada injetando-se contraste a partir do SPC para opacificar a uretra posterior, enquanto se injeta pelo meato para opacificar a uretra anterior.
A OIU pode ser vantajosa tanto para o manejo de estenoses anulares da uretra membranosa após lesões uretrais parciais quanto para estenoses curtas não obliterativas após falha do reparo anastomótico primário pós-traumático.27 O entendimento atual é que a dilatação uretral e a OIU para PFPUDD não são aceitáveis em crianças; pois os resultados relatados têm sido desfavoráveis, e os pacientes submetidos a esses procedimentos frequentemente necessitam de operações cirúrgicas adicionais.23,28
Uretroplastia anastomótica
Uretroplastia anastomótica é atualmente a abordagem preferida para restauração da continuidade uretral em crianças e adultos com PFPUDDs. O sucesso da uretroplastia anastomótica frequentemente depende de exposição cirúrgica adequada, excisão de todo o tecido fibroso que ocupa o defeito de distração, mobilização da uretra bulbar normal, fixação de mucosa saudável nas margens das extremidades uretrais bulbar e prostática e realização de uma anastomose espatulada sem tensão (Figura 7), (Figura 8), quando há suprimento sanguíneo adequado através da uretra. O reparo anastomótico pode ser tentado por muitas abordagens: (a) abordagem perineal, (b) acesso perineal elaborado em 1 estágio, (c) abordagem transpúbica (parcial ou total), (d) abordagem perineoabdominal progressiva (transpúbica), e (e) acesso sagital posterior.
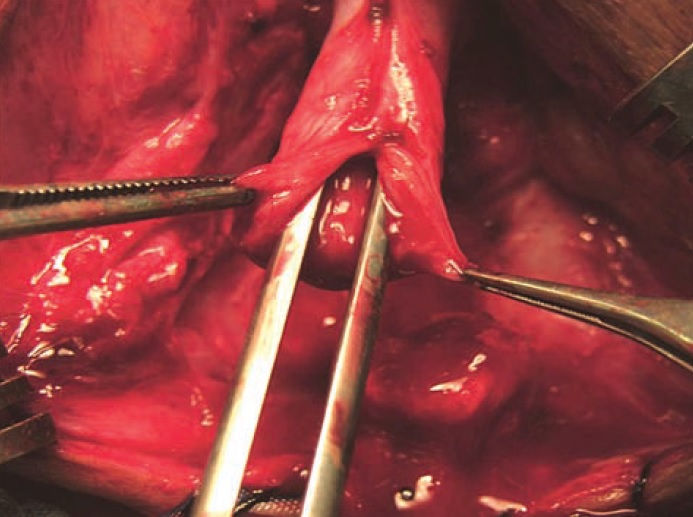
Figura 7 Mobilização adequada da uretra bulbar e anastomose ampla após espatulação são etapas cruciais
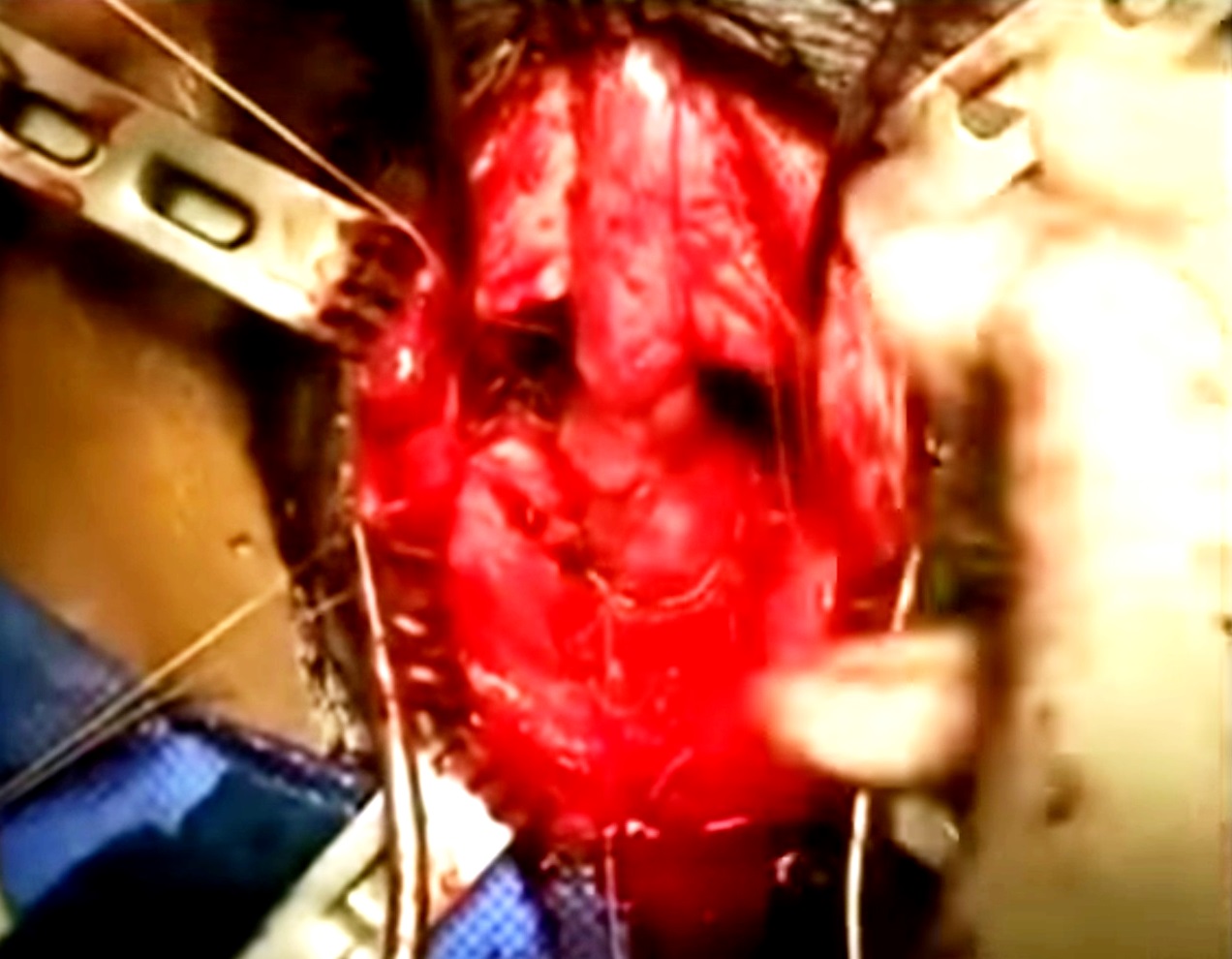
Figura 8 Mobilização das cruras e desbastamento do ramo púbico inferior contribuem para um trajeto mais curto e melhor alinhamento
A abordagem períneo-abdominal (transpúbica) permite a progressão de um acesso perineal para um acesso períneo-abdominal, com ou sem pubectomia parcial, de acordo com as características anatômicas intraoperatórias do defeito de distração uretral e permite o redirecionamento supracrural da uretra mobilizada, se necessário. O acesso perineal elaborado em 1 tempo fornece manobras em etapas para realizar uma anastomose sem tensão: (a) mobilização adequada da uretra bulbar, (b) separação dos corpos cavernosos proximais, (c) ressecção da margem inferior do arco púbico, e (d) a possibilidade de redirecionar a uretra anterior ao redor de um dos corpos cavernosos para encurtar o trajeto da uretra mobilizada
Relata-se uma taxa de sucesso global de 75-85% para a uretroplastia anastomótica.29 A falha da uretroplastia perineal foi atribuída à seleção inadequada dos pacientes - defeitos de distração de pelo menos 3 cm de comprimento com deslocamento cefálico significativo da próstata. Em crianças com PFPUDD, o reparo cirúrgico deve começar por uma exposição perineal e, quando uma anastomose sem tensão não for possível, é necessária uma abordagem abdominal (pubectomia parcial) para a correção do defeito de distração.21
Conclusões
Causas iatrogênicas (cateterização, PUV, correção de hipospádia) são responsáveis pela maioria das estenoses uretrais anteriores na faixa etária mais jovem; enquanto, à medida que a criança cresce, estenoses uretrais traumáticas e posteriores passam a predominar. O tratamento inicial das lesões traumáticas da uretra posterior associadas a fraturas pélvicas deve objetivar a estabilização do paciente, SPC e o tratamento das lesões associadas com risco de vida. A avaliação pré-operatória do defeito de distração uretral estabelecido inclui uretrograma em oposição e cistoscopia para definir a extensão anatômica do defeito de distração uretral. Quando há uma uretra anterior saudável, a uretroplastia anastomótica é ideal para tratar PFPUDDs. As uretroplastias de substituição com enxerto inlay de BMG/OMG são indicadas principalmente para estenoses panuretrais.
Referências
- Herle K, Jehangir S, Thomas RJ. Stricture Urethra in Children: An Indian Perspective. J Indian Assoc Pediatr Surg 2018; 23 (4): 192–197, DOI: 10.4103/jiaps.JIAPS_146_17.
- Ansari MS, Yadav P, Srivastava A, Kapoor R, Shekar PA. Etiology and characteristics of pediatric urethral strictures in a developing country in the 21st century. J Pediatr Urol 2019; 15 (4): 403 1–403 8, DOI: 10.1016/j.jpurol.2019.05.020.
- Harshman MW, Cromie WJ, Wein AJ, Duckett JW. Urethral Stricture Disease in Children. J Urol 1981; 126 (5): 650–654, DOI: 10.1016/S0022-5347(17)54675-3.
- Kaplan GW, Brock WA. Urethral Strictures in Children. J Urol 1983; 129 (6): 1200–1203, DOI: 10.1016/S0022-5347(17)52641-5.
- Mori Y. Treatment of congenital urethral stenosis (urethral ring) in children. Optic internal urethrotomy in the congenital bulbar urethral stenosis in boys. Nihon Hinyokika Gakkai Zasshi 1989; 80 (5): 704–710, DOI: 10.5980/jpnjurol1989.80.704.
- Gobbi D, Leon FF, Gnech M, Midrio P, Gamba P, Castagnetti M. Management of Congenital Urethral Strictures In Infants. Case Series. Urol J 2019; 16 (1): 67–71, DOI: 10.22037/uj.v0i0.4045.
- Lal R, Bhatnagar V, Mitra DK. Urethral strictures after fulguration of posterior urethral valves. J Pediatr Surg 1998; 33 (3): 518–519, DOI: 10.1016/S0022-3468(98)90102-6.
- Myers DA, Walker RD. Prevention of Urethral Strictures in the Management of Posterior Urethral Valves. J Urol 1981; 126 (5): 655–656, DOI: 10.1016/S0022-5347(17)54676-5.
- Babu R, Kumar R. Early outcome following diathermy versus cold knife ablation of posterior urethral valves. J Pediatr Urol 2013; 9 (1). DOI: 10.1016/j.jpurol.2012.02.014.
- Payne CE, Sumfest JM, Deshon GEJ. Buccal mucosal graft for hypospadias repairs. Tech Urol 1998; 4 (4): 173–176.
- Barbagli G. Correlation Between Primary Hypospadias Repair and Subsequent Urethral Strictures in a Series of 408 Adult Patients. Eur Urol Focus 2017; 3 (2–3): 287–292, DOI: 10.1016/j.euf.2017.02.005.
- Ye W-J, Ping P, Liu Y-D, Li Z, Huang Y-R. Single stage dorsal inlay buccal mucosal graft with tubularized incised urethral plate technique for hypospadias reoperations. Asian J Androl 2008; 10 (4): 682–686, DOI: 10.1111/j.1745-7262.2008.00398.x.
- Schwentner C. Interim outcome of the single stage dorsal inlay skin graft for complex hypospadias reoperations. J Urol 2006; 175 (5): 1872–1877, DOI: 10.1016/S0022-5347(05)01016-5.
- Palminteri E, Berdondini E, Verze P, Nunzio C, Vitarelli A, Carmignani L. Contemporary urethral stricture characteristics in the developed world. Urology 2013; 81 (1): 191–196, DOI: 10.1016/j.urology.2012.08.062.
- Celis S. Balanitis xerotica obliterans in children and adolescents: A literature review and clinical series. J Pediatr Urol 2014; 10 (1): 34–39, DOI: 10.1016/j.jpurol.2013.09.027.
- Dubey D, Kumar A, Mandhani A, Srivastava A, Kapoor R, Bhandari M. Buccal mucosal urethroplasty: a versatile technique for all urethral segments. BJU Int 2005; 95 (4): 625–629, DOI: 10.1111/j.1464-410X.2005.05352.x.
- Barbagli G, Sansalone S, Kulkarni SB, Romano G, Lazzeri M. Dorsal onlay oral mucosal graft bulbar urethroplasty. BJU Int 2012; 109 (11): 1728–1741, DOI: 10.1111/j.1464-410X.2012.11006.x.
- Dubey D. Substitution urethroplasty for anterior urethral strictures: a critical appraisal of various techniques. BJU Int 2003; 91 (3): 215–218, DOI: 10.1046/j.1464-410x.2003.03064.x.
- Heinke T, Gerharz EW, Bonfig R, Riedmiller H. Ventral onlay urethroplasty using buccal mucosa for complex stricture repair. Urology 2003; 61 (5): 1004–1007, DOI: 10.1016/s0090-4295(02)02523-2.
- Turner-Warwick R. Prevention of complications resulting from pelvic fracture urethral injuries–and from their surgical management. Urol Clin North Am 1989; 16 (2): 335–358. DOI: 10.1016/s0094-0143(21)01515-9.
- Podesta M, Podesta MJ. Traumatic Posterior Urethral Strictures in Children and Adolescents. Front Pediatr 2019; 7: 24, DOI: 10.3389/fped.2019.00024.
- Hagedorn JC, Voelzke BB. Pelvic-fracture urethral injury in children. Arab J Urol 2015; 13 (1): 37–42, DOI: 10.1016/j.aju.2014.11.007.
- Koraitim MM. Posttraumatic posterior urethral strictures in children: a 20-year experience. J Urol 1997; 157 (2): 641–645. DOI: 10.1016/s0022-5347(01)65239-x.
- Colapinto V, McCallum RW. Injury to the male posterior urethra in fractured pelvis: a new classification. J Urol 1977; 118 (4): 575–580, DOI: 10.1016/s0022-5347(17)58110-0.
- Goldman SM, Sandler CM, Corriere JNJ, McGuire EJ. Blunt urethral trauma: a unified, anatomical mechanical classification. J Urol 1997; 157 (1): 85–89, DOI: 10.1016/s0022-5347(01)65291-1.
- Nerli RB, Koura AC, Ravish IR, Amarkhed SS, Prabha V, Alur SB. Posterior urethral injury in male children: long-term follow up. J Pediatr Urol 2008; 4 (2): 154–159, DOI: 10.1016/j.jpurol.2007.11.002.
- Helmy TE, Hafez AT. Internal urethrotomy for recurrence after perineal anastomotic urethroplasty for posttraumatic pediatric posterior urethral stricture: could it be sufficient? J Endourol 2013; 27 (6): 693–696, DOI: 10.1089/end.2012.0592.
- Hsiao KC. Direct vision internal urethrotomy for the treatment of pediatric urethral strictures: analysis of 50 patients. J Urol 2003; 170 (3): 952–955, DOI: 10.1097/01.ju.0000082321.98172.4e.
- Singla M. Posttraumatic Posterior Urethral Strictures in Children—Management and Intermediate-term Follow-up in Tertiary Care Center. Urology 2008; 72 (3): 540–543, DOI: 10.1016/j.urology.2008.02.078.
Ultima atualização: 2025-09-21 13:35