63: Mejora de la calidad en urología pediátrica
Este capítulo durará aproximadamente 17 minutos para leer.
Introducción
En 1999, el Instituto de Medicina subrayó la necesidad de autorreflexión y de mejorar las prácticas médicas al informar que los errores prevenibles dieron lugar a casi 98 000 muertes anuales. Esta revelación contundente destacó la importancia de evaluar los resultados de los pacientes y diseñar estrategias para mejorar los procesos médicos y proporcionar una atención segura, eficaz y equitativa.1 En las últimas décadas, se ha hecho cada vez más hincapié en la mejora de la calidad (QI) y la seguridad del paciente, lo cual se ha integrado en la educación médica y en las prácticas hospitalarias. Aunque la adopción inicial ha sido lenta, estas iniciativas de mejora de la calidad se están generalizando en urología pediátrica. Este capítulo ofrecerá una breve visión general de diversas iniciativas de QI en urología pediátrica y de la metodología de QI. Es importante señalar que las diversas iniciativas de QI mencionadas a continuación no son exhaustivas, y muchos centros pueden tener proyectos de QI que no se describen.
Iniciativas de mejora de la calidad en la atención al paciente
Torsión testicular
La torsión testicular es una emergencia urológica pediátrica que se estima afecta a 3,8 por cada 100.000 niños por año. Las orquiectomías ocurren en el 41,9% de los casos en los que los testículos torsionados no son viables.2 Entre los factores que contribuyen a las orquiectomías se incluyen las demoras en la presentación, el diagnóstico, el transporte y la intervención quirúrgica. Muchas instituciones han implementado vías de atención de mejora de la calidad (QI) para agilizar el estudio diagnóstico de los pacientes que se presentan con torsiones testiculares, con la esperanza de reducir el tiempo hasta la intervención.
En 2012, Children’s Medical Center Dallas implementó una vía directa al quirófano (STOR) en la que los casos de torsión testicular diagnosticada ecográficamente procedentes de centros externos se saltarían el servicio de urgencias (ER) (Figura 1). Estos pacientes son llevados al área perioperatoria para evaluación tras una conversación de médico a médico entre el urólogo pediatra de guardia y el médico de urgencias. Con la activación de la vía, se moviliza el equipo del quirófano y, si tras la reevaluación por el equipo de urología pediátrica está indicada la cirugía, se obtienen los consentimientos, y el paciente es llevado al quirófano dentro de una hora de su llegada. La vía STOR disminuyó significativamente el tiempo mediano desde la llegada hasta la incisión de 94 min a 54 min, pero solo el 46.8% de los pacientes se sometió a intervención quirúrgica dentro de las 6 horas del inicio de los síntomas. Por lo tanto, las tasas de pérdida testicular no fueron significativamente diferentes en comparación con las tasas previas a la implementación de la vía. Se necesitan mejoras adicionales del proceso para reducir el tiempo de isquemia antes del traslado para el salvamento testicular.3
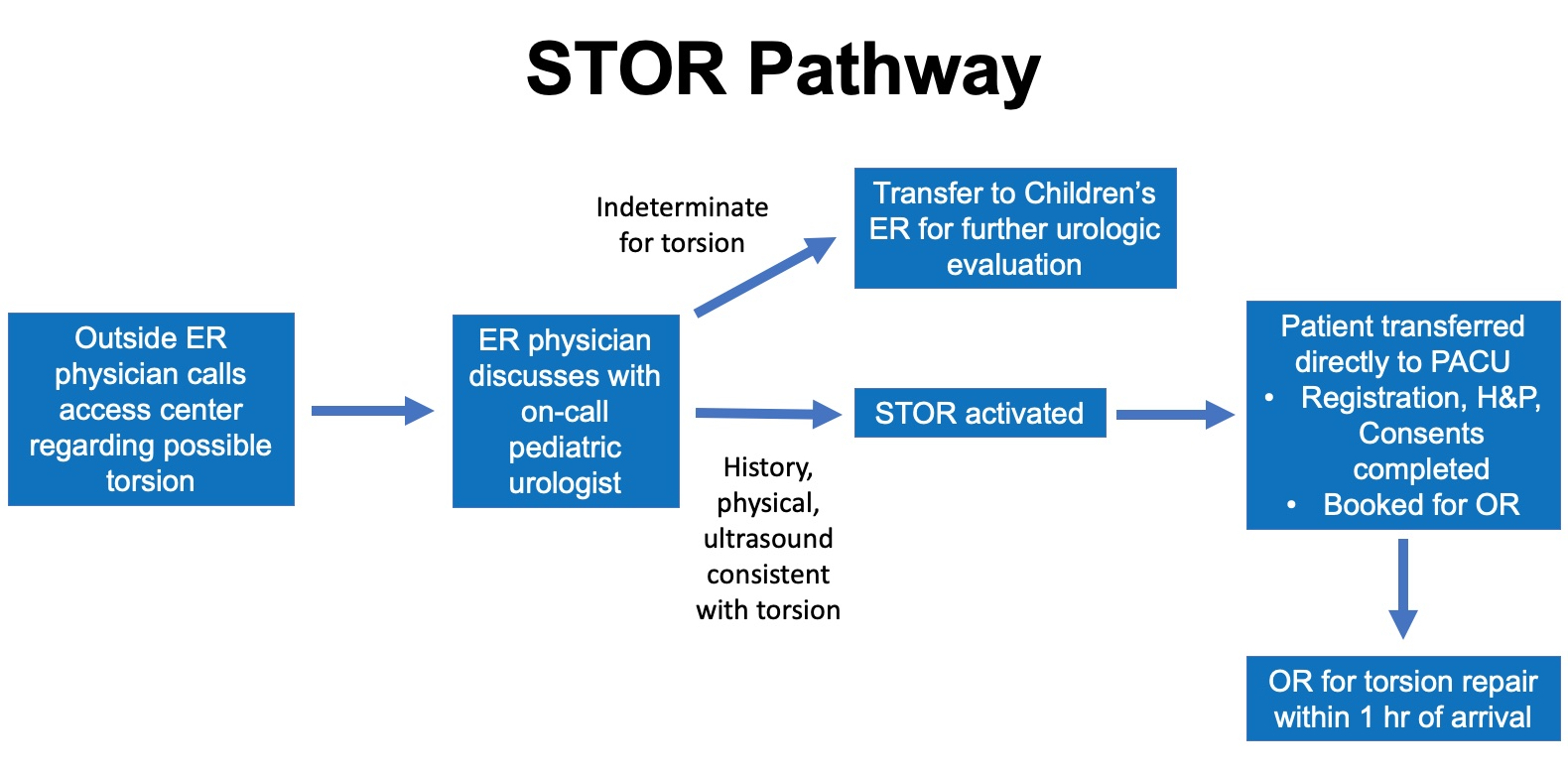
Figura 1 Vía STOR. Diagrama de la vía directa al quirófano para el manejo de la torsión en el Children’s Medical Center Dallas. Los casos de torsión confirmados por ecografía se transfieren directamente al área perioperatoria en preparación para la intervención quirúrgica. Con esta vía, se omite el Servicio de Urgencias (ER) para reducir el tiempo hasta el quirófano. Los casos indeterminados se transfieren a Urgencias para una evaluación urológica adicional. (Servicio de Urgencias - ER; Unidad de cuidados postanestésicos – PACU; Historia clínica y examen físico – H&P; Quirófano – OR)
Zee et al comunicaron su experiencia al crear una vía de atención acelerada de la torsión (ACT) para agilizar el tiempo hasta la intervención quirúrgica de las torsiones que se presentaron en el servicio de urgencias del Children’s National Medical Center. La vía disminuyó el tiempo desde el triaje en urgencias hasta el quirófano de una mediana de 196 min a 127 min. Sin embargo, las tasas de orquiectomía no cambiaron, lo que sugiere que, a pesar de reducir el tiempo desde urgencias hasta el quirófano, se necesita más trabajo para disminuir el tiempo total de isquemia testicular.4
Muchas instituciones, entre ellas Children’s Hospital of Philadelphia, Children’s Hospital Colorado y otras, han creado protocolos de manejo para sus profesionales del servicio de urgencias con el fin de orientar el abordaje diagnóstico de niños que se presentan con escroto agudo. Estos protocolos ayudan a agilizar el diagnóstico de la torsión testicular. Sin embargo, se necesitan esfuerzos para aumentar el conocimiento público sobre los signos y síntomas de la torsión testicular, a fin de reducir el tiempo desde el inicio de los síntomas hasta la evaluación médica.
Recuperación mejorada después de la cirugía
Las vías de Recuperación Mejorada Tras la Cirugía (ERAS) promueven enfoques estandarizados y basados en la evidencia para la atención posoperatoria y tienen como objetivo mejorar los procesos de recuperación de los pacientes después de cirugías mayores. Estas vías se han adoptado ampliamente en la atención quirúrgica de adultos y se ha demostrado que reducen la duración de la estancia posoperatoria, las tasas de complicaciones y los costos.5,6 En comparación, la implementación de ERAS en la cirugía pediátrica ha sido más lenta, ya que su eficacia no está bien estudiada en niños. La creencia de que ERAS aportaría un beneficio mínimo en los procedimientos pediátricos, que en general presentan baja morbilidad y mortalidad, también ha ralentizado el desarrollo y la adopción de protocolos pediátricos ERAS estandarizados.7 En la última década, esta percepción ha ido cambiando, y la implementación de ERAS ha aumentado en niños con la esperanza de mejorar su recuperación posoperatoria tras cirugías mayores.8,9 Una encuesta a urólogos pediátricos sobre ERAS mostró que el 54% informó implementar diversos elementos de ERAS en la atención posoperatoria, aunque solo el 20% reporta contar con un protocolo establecido en sus respectivas instituciones.10
En urología pediátrica, se han implementado vías ERAS en múltiples instituciones para mejorar la recuperación de los niños sometidos a reconstrucción vesical, incluidos el aumento vesical, la reconstrucción del cuello vesical o la creación de conductos cateterizables. Rove et al informaron su experiencia con ERAS en Children’s Hospital Colorado y señalaron una disminución en la duración de la estancia posoperatoria de 8 a 5.7 días en comparación con una cohorte histórica sin ERAS. También informaron una reducción del 30% en el riesgo de complicaciones en el grupo ERAS (OR 0.71, IC del 95% 0.51–0.97).11 De manera similar, Chu et al también señalaron que la duración de la estancia de los niños sometidos a reconstrucción vesical disminuyó de una mediana de 9 a 4 días tras la implementación de ERAS en Lurie Children’s Hospital, aunque no se observaron cambios significativos en las tasas de complicaciones.12 Estos estudios sugieren que ERAS puede ser beneficioso para los niños sometidos a reconstrucciones vesicales mayores. Está en curso un ensayo multicéntrico (Pediatric Urology Recovery After Surgery Endeavor) que incluye más de ocho centros en Estados Unidos y que evalúa la eficacia de ERAS en niños sometidos a reconstrucción vesical. Los resultados de este ensayo serán informativos para determinar qué elementos específicos de ERAS son críticos y ayudarán a perfeccionar futuras vías ERAS para esta población de pacientes.13 Una lista de los elementos de ERAS se muestra en Figura 2.
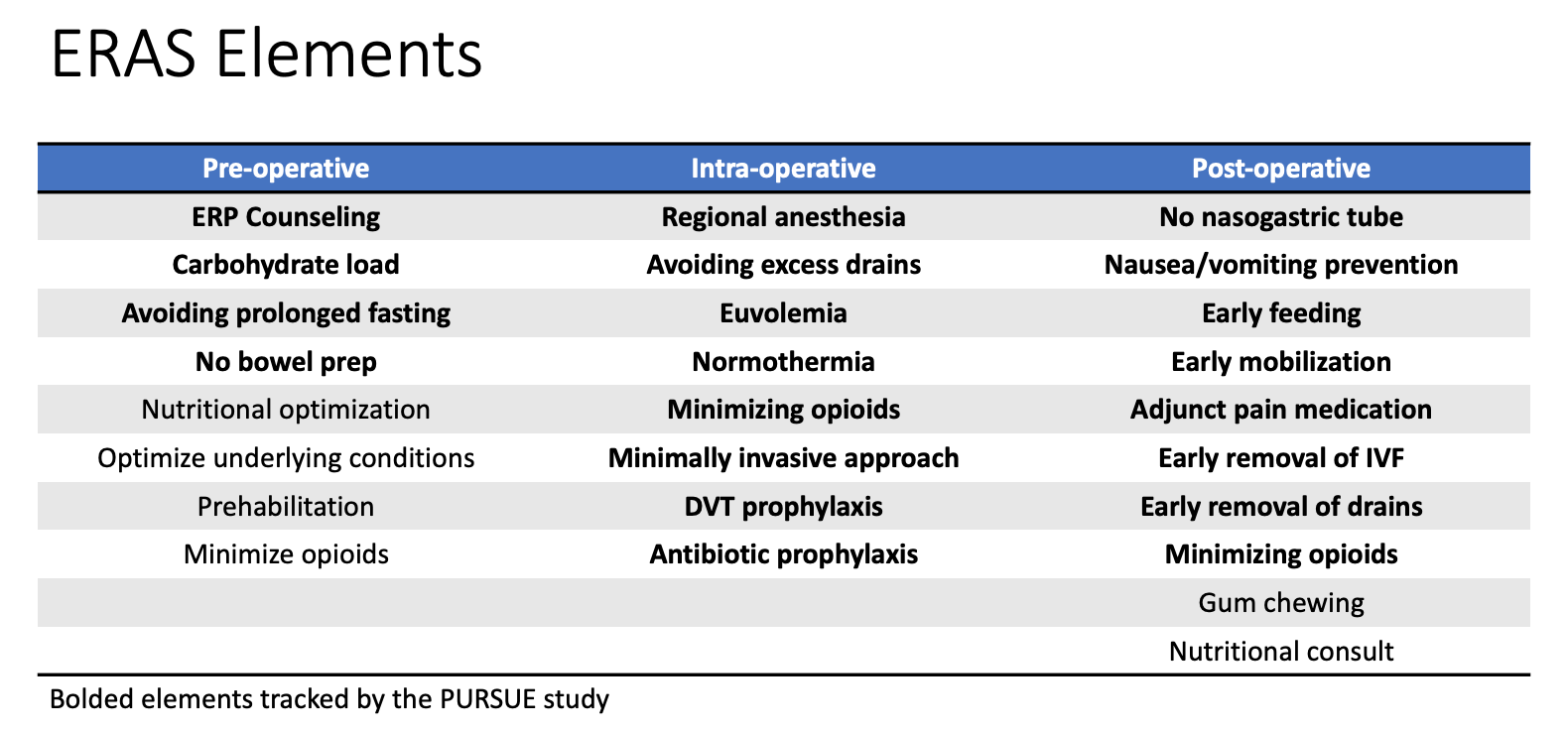
Figura 2 Elementos de ERAS. Tabla de diversos elementos de recuperación mejorada implementados como parte de las vías institucionales de Recuperación Mejorada Tras la Cirugía (ERAS). Los elementos en negrita se registran como parte del estudio multicéntrico PURSUE.
Muchos elementos de ERAS, que incluyen la alimentación precoz, la retirada precoz del catéter y la movilización precoz, ya se implementan en pacientes sometidos a reimplante ureteral y pieloplastia, lo que supone un cambio respecto a las prácticas históricas.7 Si bien no necesariamente existen protocolos estandarizados para estos casos, estos cambios en la práctica reflejan esfuerzos continuos por mejorar la atención posoperatoria de los pacientes de urología pediátrica.
Uso pediátrico de opioides
En las últimas décadas, las muertes por sobredosis de opioides han ido en aumento y fueron la causa de la mitad de las muertes pediátricas por sobredosis de fármacos entre 1999–2007.14 En respuesta a la epidemia de opioides, numerosos estudios han examinado los patrones de prescripción posoperatoria de opioides tras procedimientos urológicos pediátricos, destacando una amplia variabilidad en los hábitos de prescripción entre los proveedores.15,16 Garren et al además señalaron que el 62% de los pacientes de urología pediátrica no necesitaron ni utilizaron toda la receta de opioides que se les proporcionó.17 En respuesta a la epidemia de opioides, ha habido un esfuerzo constante entre los urólogos pediátricos para limitar las prescripciones de opioides posoperatorias.
Múltiples instituciones pediátricas han utilizado el marco de mejora de la calidad (QI) para minimizar las prescripciones de opioides en procedimientos urológicos ambulatorios. O’Kelly et al implementaron una iniciativa de QI en The Hospital for Sick Children para reducir las prescripciones de opioides en niños sometidos a reparaciones de hipospadias. La iniciativa redujo con éxito las prescripciones en un 56% y no se observaron diferencias significativas en las puntuaciones del dolor ni en los resultados postoperatorios.18 Mittal et al implementaron una iniciativa similar en el Children’s Hospital of Philadelphia para reducir las prescripciones de opioides en pacientes sometidos a procedimientos urológicos pediátricos ambulatorios. Redujeron con éxito las tasas de prescripción del 43.9% al 2.3%, con alta satisfacción de los pacientes.19 Cardona-Grau et al utilizaron el modelo Planificar-Hacer-Estudiar-Actuar (PDSA) y redujeron las prescripciones de opiáceos tras procedimientos urológicos pediátricos en un 50%. No observaron diferencias en las puntuaciones del dolor tras la implementación.20 Muchas otras iniciativas de este tipo están en curso, y sus éxitos probablemente se verán reforzados con mandatos estatales en respuesta a la crisis de los opiáceos.21
También se están realizando esfuerzos paralelos para mejorar la educación pública sobre la eliminación adecuada de opiáceos. Garren et al destacaron que el 78% de los pacientes no eliminó los opiáceos sobrantes y pocos recibieron asesoramiento perioperatorio sobre el almacenamiento y la eliminación adecuados.17 Butler et al también señalaron la falta de eliminación adecuada de opiáceos tras cirugías urológicas pediátricas a pesar del conocimiento de los métodos adecuados para hacerlo. Su estudio sugiere que el asesoramiento por parte de un profesional médico puede mejorar las tasas de eliminación adecuada, pero se necesitan más iniciativas de QI para evaluarlo mejor.
Clínica de transición
Los niños con espina bífida a menudo presentan necesidades médicas complejas y multidisciplinarias y están bajo el cuidado de urólogos pediátricos, cirujanos ortopédicos, neurocirujanos y pediatras de medicina física y rehabilitación o del desarrollo y del comportamiento. A medida que la atención médica ha avanzado, la esperanza de vida de esta población ha aumentado, lo que hace necesaria la transición de la atención a especialistas de adultos.22,23 Los estudios han mostrado que el 20% de los jóvenes con espina bífida continúan requiriendo la ayuda de un cuidador para la cateterización intermitente.24 La preparación para la transición se asocia con la alfabetización en salud del paciente, y son importantes los programas centrados en desarrollarla.25 Por múltiples razones, esta transición no siempre transcurre sin contratiempos. Muchos pacientes enfrentan dificultades para establecer la atención con especialistas de adultos y para manejar sus necesidades médicas en la adultez.
Aunque no existen guías establecidas, muchas instituciones han creado clínicas de transición para ayudar y guiar a los pacientes en este proceso. En el Children’s Medical Center Dallas, los pacientes con espina bífida son atendidos en una clínica de urología de transición para adultos después de cumplir los 18 años por un profesional de urología, un profesional de enfermería y un trabajador social. El grupo señaló que los jóvenes con vejiga neurógena necesitaban orientación sobre el manejo de la medicación, la gestión con compañías de seguros médicos y la administración de las finanzas personales. La participación del trabajo social y de enfermería fue vital para ayudar en la transición.26 En Halifax, Canadá, los pacientes alternan citas con clínicas de urología pediátrica y de adultos durante el primer año de transición.27 En el Children’s Hospital of Philadelphia, estos pacientes ven tanto a profesionales pediátricos como de adultos en el mismo espacio de la clínica para facilitar el desarrollo de la confianza médico-paciente en un entorno familiar y seguro.22 Existen muchos modelos de transición y muchas instituciones, incluidas las anteriores, han establecido programas para ayudar con este proceso desafiante y complejo. Sin embargo, a pesar de la presencia de clínicas de transición, algunos centros han observado tasas sorprendentemente bajas de transición exitosa, lo que sugiere la necesidad de más investigación e iniciativas de mejora de la calidad (QI) en este empeño.28
Cistitis hemorrágica
La cistitis hemorrágica puede presentarse en niños que han sido sometidos a radioterapia pélvica o que tienen trombocitopenia secundaria a quimioterapia o trasplante de médula ósea. Las formas graves de cistitis hemorrágica provocan retención urinaria, lo que conduce a un círculo vicioso de mayor distensión vesical y aumento del acúmulo de coágulos. Estos casos son difíciles de manejar y tienen un impacto muy negativo en la calidad de vida de los pacientes.
Como no existen guías, un enfoque de manejo multidisciplinario puede ser beneficioso en los casos graves. En Children’s Medical Center Dallas, se conformó en 2020 un grupo de trabajo sobre cistitis hemorrágica, integrado por urólogos, oncólogos, médicos de enfermedades infecciosas, radiólogos intervencionistas y farmacéuticos, para generar un enfoque escalonado para estos casos. Esta guía institucional se encuentra actualmente en desarrollo.
Reducción de residuos en quirófano
Reducir el desperdicio en el quirófano es cada vez más importante a medida que los costos de la atención sanitaria siguen aumentando. Muchos urólogos pediátricos han promovido la estandarización de los instrumentos quirúrgicos para reducir los costos del reprocesamiento. Koyle et al estandarizaron las bandejas de instrumentos para hernias pediátricas utilizadas por cirujanos pediátricos y urólogos. Redujeron el tiempo de procesamiento de instrumentos a la mitad. Aunque la reducción de costos no se evaluó específicamente, una encuesta a cirujanos y personal de enfermería señaló una mejora en la seguridad, la calidad y la eficiencia.29 Nast y Swords redujeron su bandeja de instrumentos menores de GU en un 39% y disminuyeron los costos anuales en $3,489.42 en el Rady Children’s Hospital. Utilizando el mismo enfoque, modificaron además otras bandejas quirúrgicas y estimaron ahorros potenciales anuales de $14,588.30 El equipo de urología de Lurie Children’s también ha reducido el número de instrumentos en las bandejas quirúrgicas utilizadas para casos inguinales y penianos mediante la metodología DMAIC (Definir, Medir, Analizar, Mejorar, Controlar), con resultados positivos (E. Johnson, comunicación personal, 29 de septiembre de 2021).
Mejora de la calidad de la formación en urología pediátrica
En urología pediátrica se enfatiza la importancia de formar a nuevos médicos en QI. Como parte del programa de subespecialización, los médicos en formación en urología pediátrica deben completar un proyecto institucional de QI. A través de este proceso experiencial, aprenden la metodología de QI y se enfrentan a los desafíos de superar las barreras institucionales para la implementación y de obtener el compromiso de las partes interesadas.31
La metodología de mejora de la calidad (QI) también se ha utilizado para mejorar la educación médica. Sharma et al implementaron una iniciativa de QI para mejorar la confianza y la pericia de los profesionales en la evaluación de la criptorquidia.32 La criptorquidia afecta al 1–3% de los varones a término y es un factor de riesgo conocido para el desarrollo de cáncer testicular. Puede provocar disminución de la fertilidad si ambos testículos están afectados. El diagnóstico oportuno es imperativo, pero la comodidad y la confianza de los médicos de atención primaria con el diagnóstico pueden ser variables. Los autores encuestaron a estudiantes de medicina, médicos de medicina familiar, pediatras, residentes de urología y médicos adjuntos pediátricos sobre la formación inicial y la autoconfianza para examinar testículos no descendidos. Posteriormente se realizó un examen supervisado, administrado por un urólogo pediatra, y una encuesta de seguimiento 3 meses después. La intervención mejoró la confianza de los profesionales en el diagnóstico y en las habilidades de exploración.32 A medida que seguimos mejorando la calidad de la educación médica y la retroalimentación, estudios similares serían útiles para identificar brechas de aprendizaje y fortalecer la formación urológica.
Infraestructura
Local
Muchas instituciones han establecido programas institucionales de mejora de la calidad (QI) y de seguridad del paciente, a medida que se reconoce cada vez más la importancia de la QI. Los consultores de QI ayudan a los profesionales de la salud a diseñar y ejecutar iniciativas de QI, organizar reuniones con los principales interesados y colaborar con especialistas en tecnología de la información para utilizar los sistemas de registro médico electrónico (EMR) de las instituciones con el fin de mantener estos esfuerzos. Con la extracción automatizada de datos a través del EMR, también se pueden realizar con mayor facilidad auditorías de los resultados de las iniciativas de QI.33
Nacional
A nivel nacional, el Colegio Americano de Cirujanos estableció el National Surgical Quality Improvement Program Pediatric (ACS NSQIP Peds) para evaluar y mejorar de manera más eficaz la atención quirúrgica pediátrica. Los participantes en NSQIP Peds proporcionan datos institucionales ajustados por riesgo sobre los resultados quirúrgicos a 30 días, que pueden utilizarse para establecer métricas de calidad. Se ha fomentado una mayor participación de la urología pediátrica.34
Metodología y enfoques de QI
Existen múltiples recursos disponibles para quienes estén interesados en profundizar sus conocimientos sobre la metodología de QI. El Instituto para la Mejora de la Atención Sanitaria (IHI) ofrece programas tanto presenciales como en línea, junto con una opción de certificación para convertirse en Profesional Certificado en Seguridad del Paciente. El Colegio Estadounidense de Cirujanos también ofrece un curso autodirigido sobre los conceptos básicos de los enfoques de QI. Varias instituciones ofrecen programas de certificado o de maestría en QI y seguridad del paciente para quienes deseen obtener títulos avanzados en el campo.
Squire 2.0
Para proporcionar un marco para informar sobre los trabajos de mejora de la calidad, la guía Standards for Quality Improvement Reporting Excellence (SQUIRE) fue introducida en 2008. Una versión revisada (SQUIRE 2.0) se introdujo en 2015. La guía alienta a los autores a exponer la naturaleza y la importancia del problema abordado, a describir en detalle cómo se realizó la intervención de manera que pueda ser reproducida por otros, y a evaluar el impacto y la sostenibilidad de la intervención.35 La adhesión a la guía SQUIRE 2.0 se fomenta cada vez más en las publicaciones de QI en urología pediátrica.36
Modelo para la Mejora y PDSA
Existen distintos marcos para la implementación de QI. Uno de estos enfoques es el Modelo para la Mejora, que fue desarrollado por Associates in Process Improvement.37 En este marco, el equipo plantea tres preguntas fundamentales que abordan el objetivo de la intervención, definen medidas para evaluar los efectos de la intervención e identifican áreas de cambio para la mejora. Posteriormente, los efectos del cambio o la intervención se prueban mediante el enfoque Planificar-Hacer-Estudiar-Actuar (Figura 3) Se implementa el cambio propuesto y se evalúan los efectos. Tras implementar la intervención a pequeña escala y evaluar los resultados, la intervención se refina y luego se implementa a una escala más amplia.37
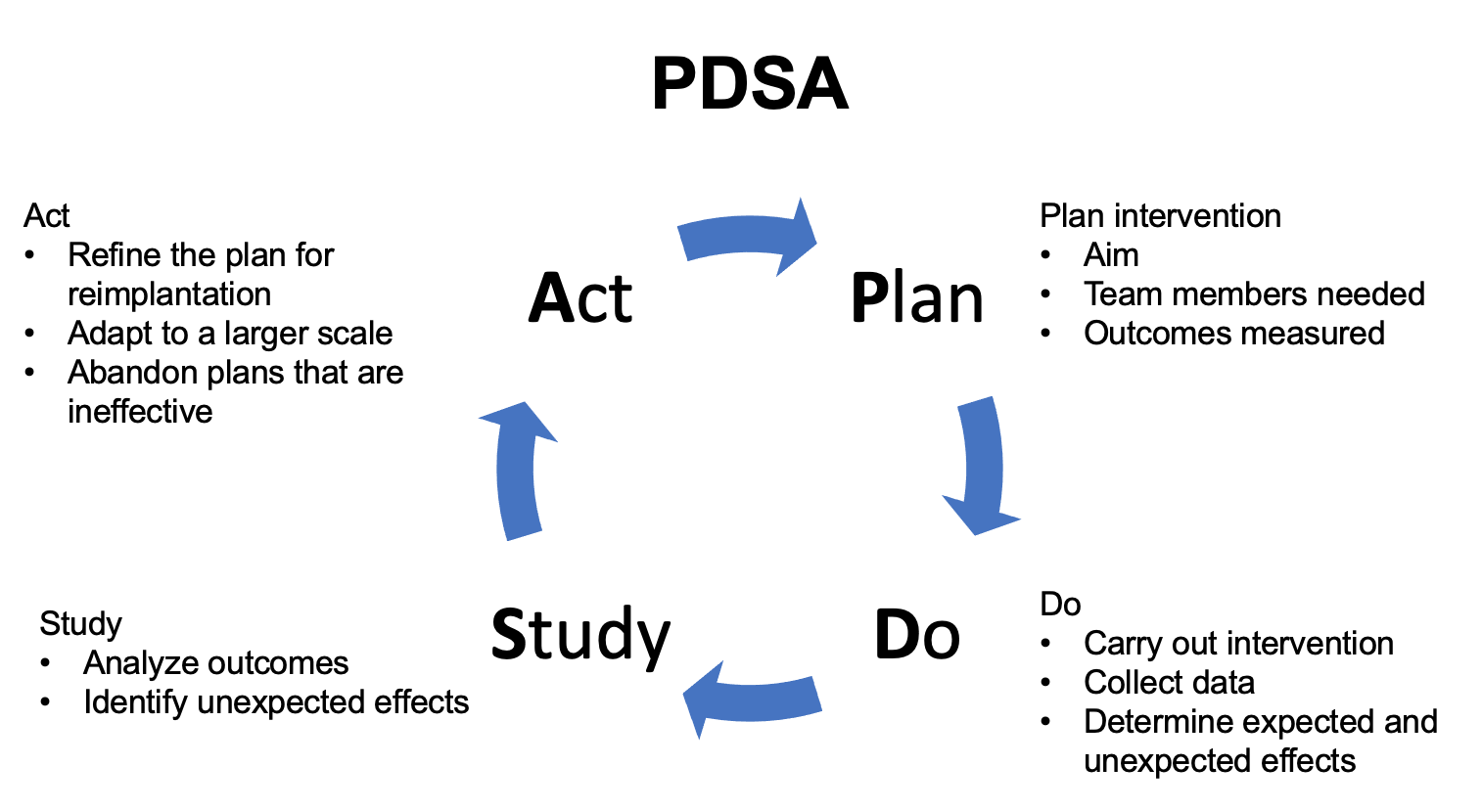
Figura 3 PDSA. Esquema que detalla el enfoque Planificar-Hacer-Estudiar-Actuar (PDSA).
Lean Six Sigma y DMAIC
Lean Six Sigma combina los enfoques del pensamiento Lean y de Seis Sigma. El pensamiento Lean se originó en la industria automotriz japonesa y se centra en reducir el desperdicio. Con este enfoque, primero se estudia el proceso que requiere mejora y se reducen los procesos derrochadores.38 Seis Sigma es un enfoque estructurado para la mejora de procesos y de la calidad que se originó en las industrias manufactureras. La combinación de los dos enfoques se conoce como Lean Six Sigma. Bajo este marco, los equipos abordan los proyectos de QI a través de cinco fases: Definir, Medir, Analizar, Mejorar, Controlar (Figura 4) En la fase Definir, se identifica el problema que se abordará. Los datos de línea de base se recopilan durante la fase Medir. En la fase Analizar, se descifra la causa raíz del problema y se implementan medidas de mejora. En la fase Controlar, se mantienen los cambios implementados.39
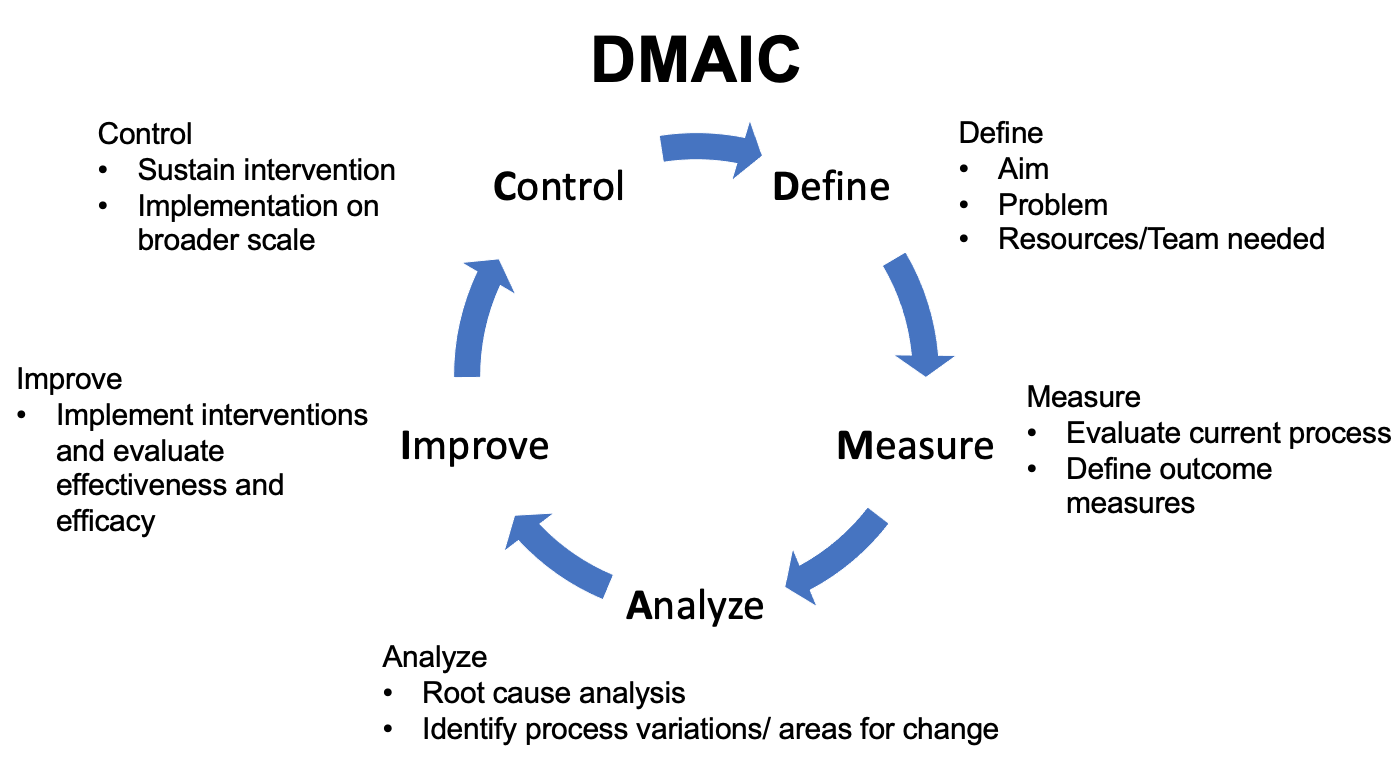
Figura 4 DMAIC. Esquema que detalla el enfoque Definir-Medir-Analizar-Mejorar-Controlar.
Direcciones futuras
La mejora de la calidad está cobrando impulso en la urología pediátrica, pero aún queda un largo camino por recorrer. En comparación con la investigación clínica estándar, la metodología, el proceso de implementación y la gestión de las barreras de implementación a nivel global e institucional son especialmente críticos para el éxito de los proyectos de QI. Si bien revistas, incluida Journal of Pediatric Urology, están publicando más artículos de QI, estas publicaciones siguen siendo escasas en la literatura urológica. Una plataforma de discusión para entusiastas de QI en urología pediátrica puede ayudar a hacer avanzar los proyectos a medida que, colectivamente, sorteamos las barreras de implementación y aprendemos de las experiencias y dificultades de los demás. La rareza relativa de las afecciones urológicas pediátricas también hace que definir “calidad” sea un desafío, ya que estamos limitados por la presencia de evidencia de alta calidad para respaldar las mejores prácticas.36 No obstante, la implementación de prácticas basadas en datos puede ayudar a evaluar mejor su eficacia y seguridad a medida que seguimos cuestionando el dogma. Como urólogos pediátricos, nuestra participación y liderazgo en QI son fundamentales mientras nos esforzamos por la mejora continua y hacemos avanzar nuestro campo.
Lecturas recomendadas
- Instituto para la Mejora de la Atención Sanitaria
- Hannick JH, O’Kelly F, Wolfstadt JI. Improving care in pediatric urology-A primer on quality improvement methodology and how to apply it to pediatric urology. J Pediatr Urol 2019; 15: 503, DOI: 10.1016/j.jpurol.2019.09.014.
- Cohen R. I.: Lean Methodology in Health Care. Chest 2018; 154: 1448, DOI: 10.1016/j.chest.2018.06.005.
- Koning H, Verver JP, Heuvel J. Lean six sigma in healthcare. J Healthc Qual 2006; 28: 4, DOI: 10.4018/978-1-4666-7320-5.ch009.
Referencias
- Kohn LT, Corrigan JM, Washington MSD, editors. To Err is Human: Building a Safer Health System. 2000. DOI: 10.1016/s1051-0443(01)70072-3.
- Zhao LC, Lautz TB, Meeks JJ. Pediatric testicular torsion epidemiology using a national database: incidence, risk of orchiectomy and possible measures toward improving the quality of care. J Urol 2011; 186: 2009, DOI: 10.1016/j.juro.2011.07.024.
- Arevalo MK, Sheth KR, Menon VS. Straight to the Operating Room: An Emergent Surgery Track for Acute Testicular Torsion Transfers. J Pediatr 2018; 192: 178, DOI: 10.1016/j.jpeds.2017.09.009.
- Zee RS, Bayne CE, Gomella PT. Implementation of the accelerated care of torsion pathway: a quality improvement initiative for testicular torsion. J Pediatr Urol 2019; 15: 473, DOI: 10.1016/j.jpurol.2019.07.011.
- Heathcote S Sr., Duggan K, Rosbrugh J. Enhanced Recovery after Surgery (ERAS) Protocols Expanded over Multiple Service Lines Improves Patient Care and Hospital Cost. Am Surg 2019; 85: 1044, DOI: 10.1177/000313481908500951.
- Ljungqvist O, Scott M, Fearon K. C.: Enhanced Recovery After Surgery: A Review. JAMA Surg 2017; 152: 292, DOI: 10.1007/978-3-030-33443-7.
- Cain MP. Enhanced Recovery after Surgery Protocols in Pediatric Urology-How are we Doing and What Should we be Doing? J Urol 2018; 200: 952, DOI: 10.1016/j.juro.2018.08.037.
- Brindle ME, McDiarmid C, Short K. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations. World J Surg 2020; 44: 2482, DOI: 10.1007/s00268-020-05530-1.
- Short HL, Heiss KF, Burch K. Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2018; 53: 688, DOI: 10.1016/j.jpedsurg.2017.05.004.
- Chan YY, Rosoklija I, Meade P. Utilization of and barriers to enhanced recovery pathway implementation in pediatric urology. J Pediatr Urol 2021; 17: 294 1, DOI: 10.1016/j.jpurol.2021.01.044.
- Rove KO, Brockel MA, Saltzman AF. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol 2018; 14: 252 1, DOI: 10.1016/j.jpurol.2018.01.001.
- Chan YY, Chu DI, Hirsch J. Implementation and sustainability of an enhanced recovery pathway in pediatric bladder reconstruction: Flexibility, commitment, teamwork. J Pediatr Urol 2021. DOI: 10.1016/j.jpurol.2021.08.023.
- Rove KO, Strine AC, Wilcox DT. Design and development of the Pediatric Urology Recovery After Surgery Endeavor (PURSUE) multicentre pilot and exploratory study. BMJ Open 2020; 10: 039035, DOI: 10.1016/j.juro.2018.02.2313.
- Kelly BC, Vuolo M, Frizzell LC. Pediatric drug overdose mortality: contextual and policy effects for children under 12 years. Pediatr Res 2021. DOI: 10.1038/s41390-021-01567-7.
- Corona LE, Roth EB, Thao A. Opioid prescribing is excessive and variable after pediatric ambulatory urologic surgery. J Pediatr Urol 2021; 17: 259 1, DOI: 10.1016/j.jpurol.2021.01.008.
- Ahn JJ, Ellison JS, Merguerian P. A.: A Societies for Pediatric Urology survey of opioid prescribing practices after ambulatory pediatric urology procedures. J Pediatr Urol 2019; 15: 451, DOI: 10.1016/j.jpurol.2019.04.025.
- Garren BR, Lawrence MB, McNaull PP. Opioid-prescribing patterns, storage, handling, and disposal in postoperative pediatric urology patients. J Pediatr Urol 2019; 15: 260 1, DOI: 10.1016/j.jpurol.2019.02.009.
- O’Kelly F, Pokarowski M, DeCotiis KN. Structured opioid-free protocol following outpatient hypospadias repair - A prospective SQUIRE 2.0-compliant quality improvement initiative. J Pediatr Urol 2020; 16: 647 1, DOI: 10.1016/j.jpurol.2020.06.012.
- Mittal S, Shukla AR, Sahadev R. Reducing post-operative opioids in children undergoing outpatient urologic surgery: A quality improvement initiative. J Pediatr Urol 2020; 16: 846 1, DOI: 10.1016/j.jpurol.2020.09.022.
- Cardona-Grau D, Bush RA, Le HK. Reducing Opioid Prescriptions in Outpatient Pediatric Urological Surgery. J Urol 2019; 201: 1012, DOI: 10.1097/ju.0000000000000020.
- Villanueva J, Pifer B, Colaco M. A government mandated consent safely reduces opioid utilization for major pediatric genitourinary surgeries. J Pediatr Surg 2021. DOI: 10.1016/j.jpedsurg.2021.01.004.
- Skokan AJ, Kovell RC. Advances and Challenges in Transitional Urology: Caring for Adolescents and Young Adults with Lifelong Complex Genitourinary Conditions. Curr Urol Rep 2018; 19: 26, DOI: 10.1007/s11934-018-0774-3.
- Hsieh MH, Wood HM, Dicianno BE. Research Needs for Effective Transition in Lifelong Care of Congenital Genitourinary Conditions: A Workshop Sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. Urology 2017; 103: 261, DOI: 10.1016/j.urology.2016.12.052.
- Chu DI, Kayle M, Stern A. Longitudinal Trajectories of Clean Intermittent Catheterization Responsibility in Youths with Spina Bifida. J Urol 2021; 101097JU0000000000002204. DOI: 10.1097/ju.0000000000002204.
- Rague JT, Kim S, Hirsch JA. Assessment of Health Literacy and Self-reported Readiness for Transition to Adult Care Among Adolescents and Young Adults With Spina Bifida. JAMA Netw Open 2021; 4: 2127034, DOI: 10.1001/jamanetworkopen.2021.27034.
- Grimsby GM, Burgess R, Culver S. Barriers to transition in young adults with neurogenic bladder. J Pediatr Urol 2016; 12: 258 1, DOI: 10.1016/j.jpurol.2016.04.015.
- Duplisea JJ, Romao RL, MacLellan DL. Urological Follow-up in Adult Spina Bifida Patients: Is There an Ideal Interval? Urology 2016; 97: 269, DOI: 10.1016/j.urology.2016.06.025.
- Szymanski KM, Cain MP, Hardacker TJ. How successful is the transition to adult urology care in spina bifida? A single center 7-year experience. J Pediatr Urol 2017; 13: 40 1, DOI: 10.1016/j.jpurol.2016.09.020.
- Koyle MA, AlQarni N, Odeh R. Reduction and standardization of surgical instruments in pediatric inguinal hernia repair. J Pediatr Urol 2018; 14: 20, DOI: 10.1016/j.jpurol.2017.08.002.
- Nast K, Swords K. A.: Decreasing operating room costs via reduction of surgical instruments. J Pediatr Urol 2019; 15: 153 1, DOI: 10.1016/j.jpurol.2019.01.013.
- Hannick JH, O’Kelly F, Wolfstadt JI. Improving care in pediatric urology-A primer on quality improvement methodology and how to apply it to pediatric urology. J Pediatr Urol 2019; 15: 503, DOI: 10.1016/j.jpurol.2019.09.014.
- Buchhalter JR, Scantlebury MH, D’Alfonso S. Creation and implementation of an electronic health record note for quality improvement in pediatric epilepsy: Practical considerations and lessons learned. Epilepsia Open 2021; 6: 345, DOI: 10.1002/epi4.12480.
- Ellison JS. Society for Pediatric Urology (SPU): NSQIP update. Societies for Pediatric Urology, Virtual. 2011.
- Ogrinc G, Davies L, Goodman D. Squire 2.0 (Standards for Quality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. Am J Crit Care 2015; 24: 466, DOI: 10.1016/s1553-7250(15)41062-1.
- O’Kelly F, Hannick JH, Wolfstadt JI. Quality improvement in pediatric urology-a historical perspective on street pumps, puerperal fever, surgical infection, and contemporary methodology. J Pediatr Urol 2019; 15: 495, DOI: 10.1016/j.jpurol.2019.08.016.
- Langley GL, R. M, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed., San Francisco, California, USA: Jossey-Bass Publishers; 2009, DOI: 10.1080/10686967.1998.11919154.
- Cohen R. I.: Lean Methodology in Health Care. Chest 2018; 154: 1448, DOI: 10.1016/j.chest.2018.06.005.
- Koning H, Verver JP, Heuvel J. Lean six sigma in healthcare. J Healthc Qual 2006; 28: 4, DOI: 10.4018/978-1-4666-7320-5.ch009.
Última actualización: 2025-09-21 13:35