63: Quality Improvement in Pediatric Urology
阅读本章大约需要 15 分钟。
Introduction
In 1999, the Institute of Medicine highlighted the need for self-reflection and improvement in medical practices when it reported that preventable errors resulted in nearly 98,000 annual deaths. This striking revelation emphasized the importance of evaluating patient outcomes and devising strategies to improve medical processes and provide safe, effective, and equitable care.1 Over the last few decades, there has been increasing emphasis on quality improvement (QI) and patient safety which has been integrated into medical education and hospital practices. Although initial uptake has been slow, these quality improvement initiatives are becoming more widespread in pediatric urology. This chapter will provide a brief overview of various QI initiatives in pediatric urology and QI methodology. It is important to note the various QI initiatives mentioned below are not comprehensive, and many centers may have QI projects that are not outlined.
Quality Improvement Initiatives in Patient Care
Testicular Torsion
Testicular torsion is a pediatric urologic emergency that affects an estimated 3.8 per 100,000 boys per year. Orchiectomies occur in 41.9% of cases in which the torsed testes are not viable.2 Contributing factors to orchiectomies include delays in presentation, diagnosis, transport, and surgical intervention. Many institutions have implemented QI care pathways to expedite workup of patients presenting with testicular torsions with hopes of reducing time to intervention.
In 2012, Children’s Medical Center Dallas implemented a straight to operating room (STOR) pathway in which cases of sonographically diagnosed torsion transfers from outside institutions would bypass the emergency room (ER) (Figure 1). These patients are brought to the perioperative area for evaluation after a physician-to-physician conversation between the on-call pediatric urologist and emergency room physician. With pathway activation, the operating room team is mobilized and if surgery is indicated after re-evaluation by the pediatric urology team, consents are obtained, and the patient is taken to the operating room within an hour of arrival. The STOR pathway significantly decreased median arrival to incision time from 94 min to 54 min, but only 46.8% of patients underwent surgical intervention within 6 hours of symptom onset. As such, rates of testicular loss were not significantly different compared to rates prior to pathway implementation. Further process improvements to reduce ischemia time prior to transfer is needed for testicular salvage.3
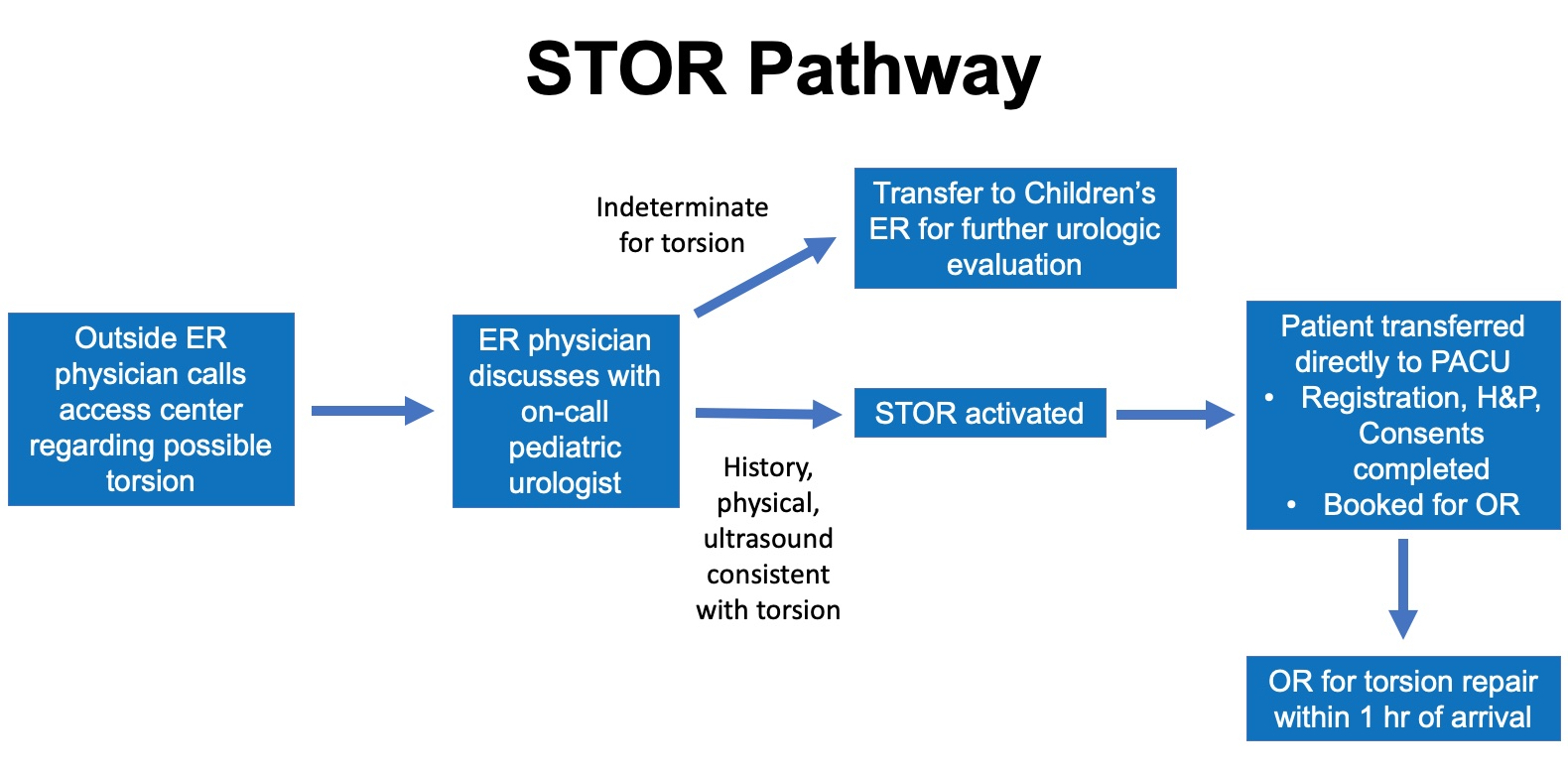
Figure 1 STOR Pathway. Diagram of the Straight to Operating Room Pathway for torsion management at Children’s Medical Center Dallas. Cases of ultrasound confirmed torsions are transferred directly to the perioperative area in preparation for surgical intervention. With this pathway, the emergency room (ER) is bypassed to reduced time to the operating room. Indeterminate cases are transferred to the ER for further urologic evaluation. (Emergency Room - ER; Post-anesthesia care unit – PACU; History and Physical – H&P; Operating Room – OR)
Zee et al reported their experience creating an accelerated care of torsion pathway (ACT) to expedite time to surgical intervention for torsions that presented at the Children’s National Medical Center ER. The pathway decreased time from ER triage to the operating room from a median of 196 min to 127 min. However, orchiectomy rates were unchanged, suggesting that despite decreasing time from ER to OR, further work is needed to decrease total testicular ischemia time.4
Many institutions including Children’s Hospital of Philadelphia, Children’s Hospital Colorado, and others have created management pathways for their ER providers to guide workup of boys presenting with acute scrotums. These pathways help expedite diagnosis of testicular torsion. However, efforts are needed to increase public knowledge of signs and symptoms of testicular torsion to reduce time from symptom onset to medical evaluation.
Enhanced Recover After Surgery
Enhanced Recovery After Surgery pathways promote standardized, evidence-based approaches to post-operative care and aim to improve patients’ recovery processes after major surgery. These pathways have been widely adopted in adult surgical care and have been shown to reduce post-operative length of stay, complication rates, and cost.5,6 In comparison, ERAS implementation in pediatric surgery has been slower, as its efficacy is not well studied in children. The belief that ERAS would yield minimal benefit for pediatric procedures, which generally have low morbidity and mortality, has also slowed development and adoption of standardized pediatric ERAS protocols.7 In the last decade, this sentiment has been changing, and ERAS implementation has increased in children with hopes of improving their post-operative recovery after major surgery.8,9 A survey of pediatric urologists regarding ERAS showed that 54% reported implementing various ERAS elements in post-operative care, although only 20% report having an established protocol at their respective institutions.10
In pediatric urology, ERAS pathways have been implemented at multiple institutions to improve recovery for children undergoing bladder reconstruction including bladder augmentation, bladder neck reconstruction, or creation of catheterizable channels. Rove et al reported their experience with ERAS at Children’s Hospital Colorado and noted a decrease in post-operative length of stay from 8 to 5.7 days when compared to a historical non-ERAS cohort. They also reported a 30% decrease in risk of complications in the ERAS group (OR 0.71, 95% CI 0.51–0.97).11 Similarly, Chu et al also noted that length of stay for children undergoing bladder reconstruction decreased from a median of 9 to 4 days following ERAS implementation at Lurie Children’s Hospital, though no significant changes in complication rates were noted.12 These studies suggest that ERAS may be beneficial for children undergoing major bladder reconstruction. A multicenter trial (Pediatric Urology Recovery After Surgery Endeavor) involving over eight centers across the United States is ongoing and is evaluating the efficacy of ERAS in children undergoing bladder reconstruction. Results from this trial will be informative in determining which specific ERAS elements are critical and will help refine future ERAS pathways for this patient population.13 A list of ERAS elements are shown in Figure 2.
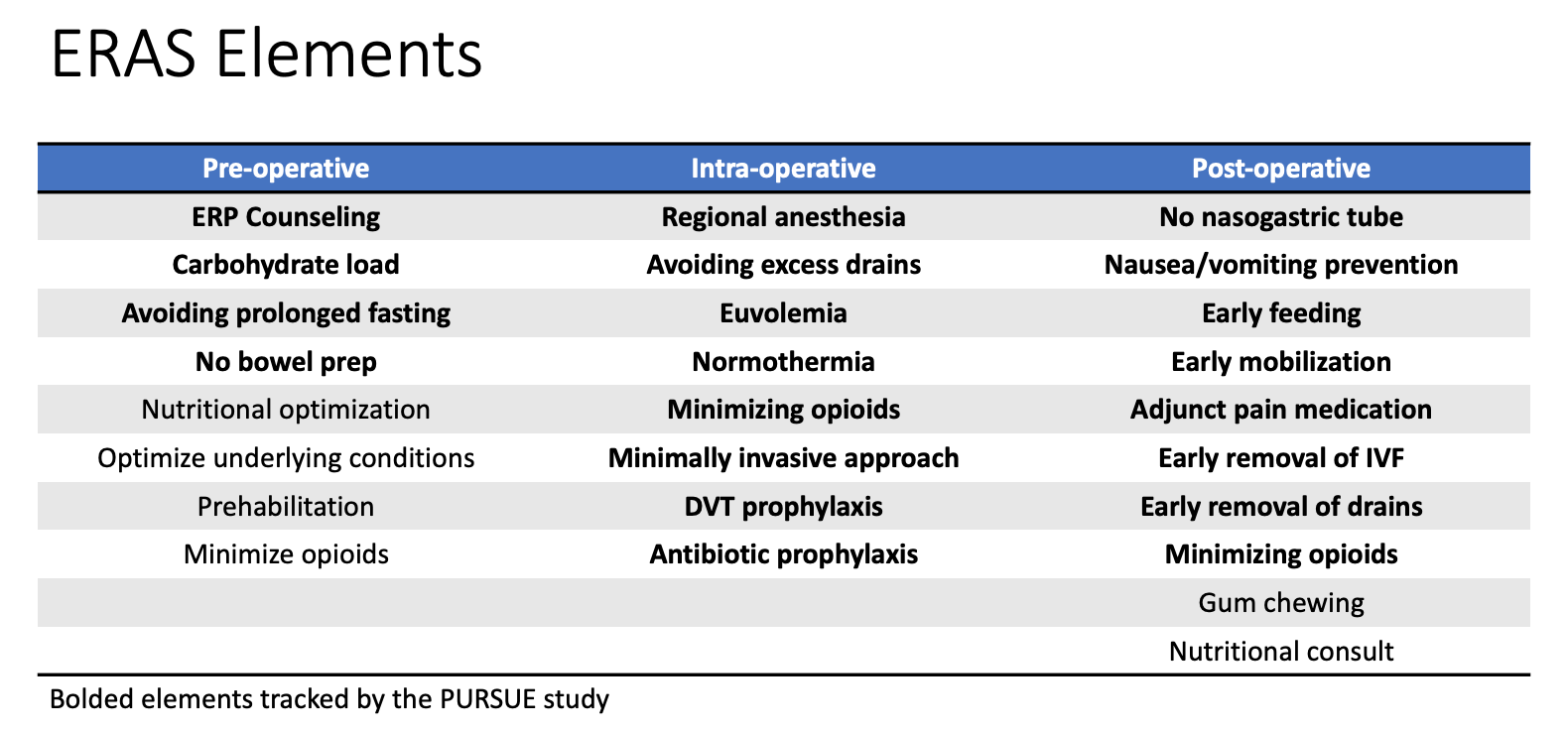
Figure 2 ERAS Elements. Table of various enhanced recovery elements implemented as a part of institutional Enhanced Recovery After Surgery (ERAS) pathways. Bolded elements are tracked as a part of the multicenter study PURSUE.
Many ERAS elements including early feeding, early catheter removal, and early mobility are already implemented for patients undergoing ureteral reimplantation and pyeloplasty, a shift from historical practices.7 While standardized protocols do not necessarily exist for these cases, these practice changes reflect ongoing efforts in improving the post-operative care of pediatric urology patients.
Pediatric Opioid Use
In the last several decades, death from opioid overdose has been increasing and is the cause of half of pediatric drug overdose mortalities from 1999–2007.14 In response to the opioid epidemic, many studies have examined post-operative opioid prescription patterns after pediatric urologic procedures, highlighting wide variability in prescription habits amongst providers.15,16 Garren et al further noted that 62% of pediatric urology patients did not need or use the entire opioid prescription provided.17 In response to the opioid epidemic, there has been steady effort amongst pediatric urologists to limit post-operative opioid prescriptions.
Multiple pediatric institutions have utilized the QI framework to minimize opioid prescriptions for outpatient urologic procedures. O’Kelly et al implemented a QI initiative at The Hospital for Sick Children to reduce opioid prescriptions for children undergoing hypospadias repairs. The initiative successfully reduced prescriptions by 56% and noted no significant difference in pain scores or post-operative outcomes.18 Mittal et al implemented a similar initiative at Children’s Hospital of Philadelphia to reduce opioid prescriptions for patients undergoing ambulatory pediatric urologic procedures. They successfully reduced prescription rates from 43.9% to 2.3% with high patient satisfaction.19 Cardona-Grau et al used the Plan-Do-Study-Act (PDSA) model and reduced opiate prescriptions following pediatric urologic procedures by 50%. They noted no differences in pain scores following implementation.20 Many other such initiatives are ongoing, and their successes will likely be augmented with state mandates in response to the opiate crisis.21
Concurrent efforts are also being made to improve public education of proper opiate disposal. Garren et al highlighted that 78% of patients did not dispose of excess opiates and few received perioperative counseling regarding proper storage and disposal.17 Butler et al also noted lack of proper opiate disposal following pediatric urologic surgeries despite knowledge of proper methods of doing so. Their study suggests that counseling by a medical professional may improve proper disposal rates, but further QI initiatives are needed to better evaluate this.
Transitional Clinic
Children with spina bifida often have complex multidisciplinary medical needs and are under the care of pediatric urologists, orthopedic surgeons, neurosurgeons, and physical medicine and rehabilitation or developmental and behavioral pediatricians. As medical care has advanced, life expectancy for this population has increased, necessitating the transition of care to adult specialists.22,23 Studies have shown that 20% of youths with spina bifida continue to require caregiver assistance with intermittent catheterization.24 Readiness to transition is associated with patient health literacy, and programs focused on developing this is important.25 Due to multiple reasons, this transition may not always go smoothly. Many patients face issues of establishing care with adult specialists and managing their medical needs in adulthood.
Although no set guidelines exist, many institutions have established transitional clinics to assist and guide patients in this process. At Children’s Medical Center Dallas, patients with spina bifida are seen in an adult transitional urology clinic after they reach 18 years of age by a urology provider, nurse, and a social worker. The group noted that youth with neurogenic bladder needed guidance with medication management, navigating medical insurance companies, and managing personal finance. Social work and nursing involvement were vital in assisting with the transition.26 In Halifax, Canada, patients alternate appointments with pediatric and adult urology clinics during the first year of transition.27 At Children’s Hospital of Philadelphia, these patients see both pediatric and adult providers in the same clinic space to facilitate the development of physician-patient trust in a familiar and safe environment.22 Many models for transition exist and many institutions including those above have established programs to assist with this challenging and complex process. However, despite the presence of transition clinics, some centers have noted surprising low rates of successful transition, suggesting need for more research and QI initiatives in this endeavor.28
Hemorrhagic Cystitis
Hemorrhagic cystitis can occur in children who have undergone pelvic radiation or who have thrombocytopenia secondary to chemotherapy or bone marrow transplant. Severe forms of hemorrhagic cystitis result in urinary retention, leading to a vicious cycle of further bladder distention and increasing clot burden. These cases are challenging to manage and have severely negative impacts on the patients’ quality of life.
As no guidelines exist, a multidisciplinary management approach may be beneficial in severe cases. At Children’s Medical Center Dallas, a hemorrhagic cystitis working group consisting of urologists, oncologists, infectious disease physicians, interventional radiologists, and pharmacists was assembled in 2020 to generate a stepwise approach to these cases. This institutional guideline is currently under development.
Reducing OR Waste
Reducing waste in the operating room is increasingly important as healthcare costs continue to rise. Many pediatric urologists have promoted standardization of surgical instruments to reduce costs of re-processing. Koyle et al standardized the instrument trays for pediatric hernias used by pediatric surgeons and urologists. They decreased the instrument processing time by half. Although cost reduction was not specifically evaluated, a survey of surgeons and nurses noted improvement in safety, quality, and efficiency.29 Nast and Swords reduced their GU minor instrument tray by 39% and decreased annual costs by $3,489.42 at Rady Children’s Hospital. Using the same approach they further modified other surgical trays and estimated annual potential savings to be $14,588.30 The urology team at Lurie Children’s has also reduced the number of instruments in the surgical trays utilized for groin and penile cases using the DMAIC methodology (Define, Measure, Analyze, Improve, Control) with positive results (E. Johnson, personal communication, September 29, 2021).
Quality Improvement in Pediatric Urology Training
The importance of training new physicians in QI is emphasized in pediatric urology. As a part of fellowship, pediatric urology fellows are required to complete an institutional QI project. Through this experiential process, they learn QI methodology and experience the challenges of overcoming institutional implementation barriers and acquiring stakeholder buy-in.31
QI methodology has also been utilized in improving medical education. Sharma et al implemented a QI initiative to improve provider confidence and expertise in evaluating cryptorchidism.32 Cryptorchidism affects 1–3% of term boys and is a known risk factor for testicular cancer development. It can result in decreased fertility if both testes are affected. Timely diagnosis is imperative, but primary care physicians’ comfort and confidence with diagnosis may be variable. The authors surveyed medical students, family medicine practitioners, pediatricians, urology residents and pediatric attendings on baseline training and self confidence in examining undescended testes. This was followed by a proctored exam administered by a pediatric urologist and a follow up survey 3 months later. The intervention improved provider confidence in diagnosis and examination skills.32 As we continue to improve quality of medical education and feedback, similar studies would be helpful to identify learning gaps and enhance urologic training.
Infrastructure
Local
Many institutions have established institutional QI and patient safety programs as the importance of QI is increasingly recognized. QI consultants help providers design and execute QI initiatives, organize major stakeholder meetings, and collaborate with information technology specialists to use the institutions’ electronic medical record (EMR) systems to sustain these efforts. With automated data extraction through the EMR, audits of QI initiative outcomes can also be more easily performed.33
National
On a national level, the American College of Surgeons established the National Surgical Quality Improvement Program Pediatric (ACS NSQIP Peds) to better assess and improve surgical care for children. Participants in NSQIP Peds provide institutional risk-adjusted data on 30-day surgical outcomes which may be used to establish quality metrics. Increasing pediatric urology participation has been encouraged.34
Methodology and Approaches to QI
Multiple resources are available for those interested in further advancing their knowledge on QI methodology. The Institute for Healthcare Improvement (IHI) offers both in-person and online programs along with a credentialing option to become a Certified Professional in Patient Safety. The American College of Surgeons also offers a self-paced course on the basics of QI approaches. Various institutions offer certificate or Master programs in QI and patient safety for those wishing to pursue advanced degrees in the field.
Squire 2.0
To provide a framework for reporting quality improvement work, the Standards for Quality Improvement Reporting Excellence (SQUIRE) guideline was introduced in 2008. A revised version (SQUIRE 2.0) was introduced in 2015. The guideline encourages authors to expound on the nature and significance of the problem addressed, outline in detail how the intervention was performed such that it is reproducible by others, and assess the intervention’s impact and sustainability.35 Adherence to the SQUIRE 2.0 guideline is increasingly encouraged with QI publications in pediatric urology.36
Model for Improvement and PDSA
Different frameworks exist for QI implementation. One such approach is the Model for Improvement which was developed by Associates in Process Improvement.37 Under this framework, the team asks three fundamental questions addressing the aim of the intervention, defining measures to evaluate the effects of the intervention, and identifying areas of change for improvement. The effects of the change or intervention are then tested via the Plan-Do-Study-Act approach (Figure 3) The proposed change is implemented, and the effects assessed. After implementing the intervention on a small scale and evaluating the results, the intervention is then refined and then implemented on a broader scale.37
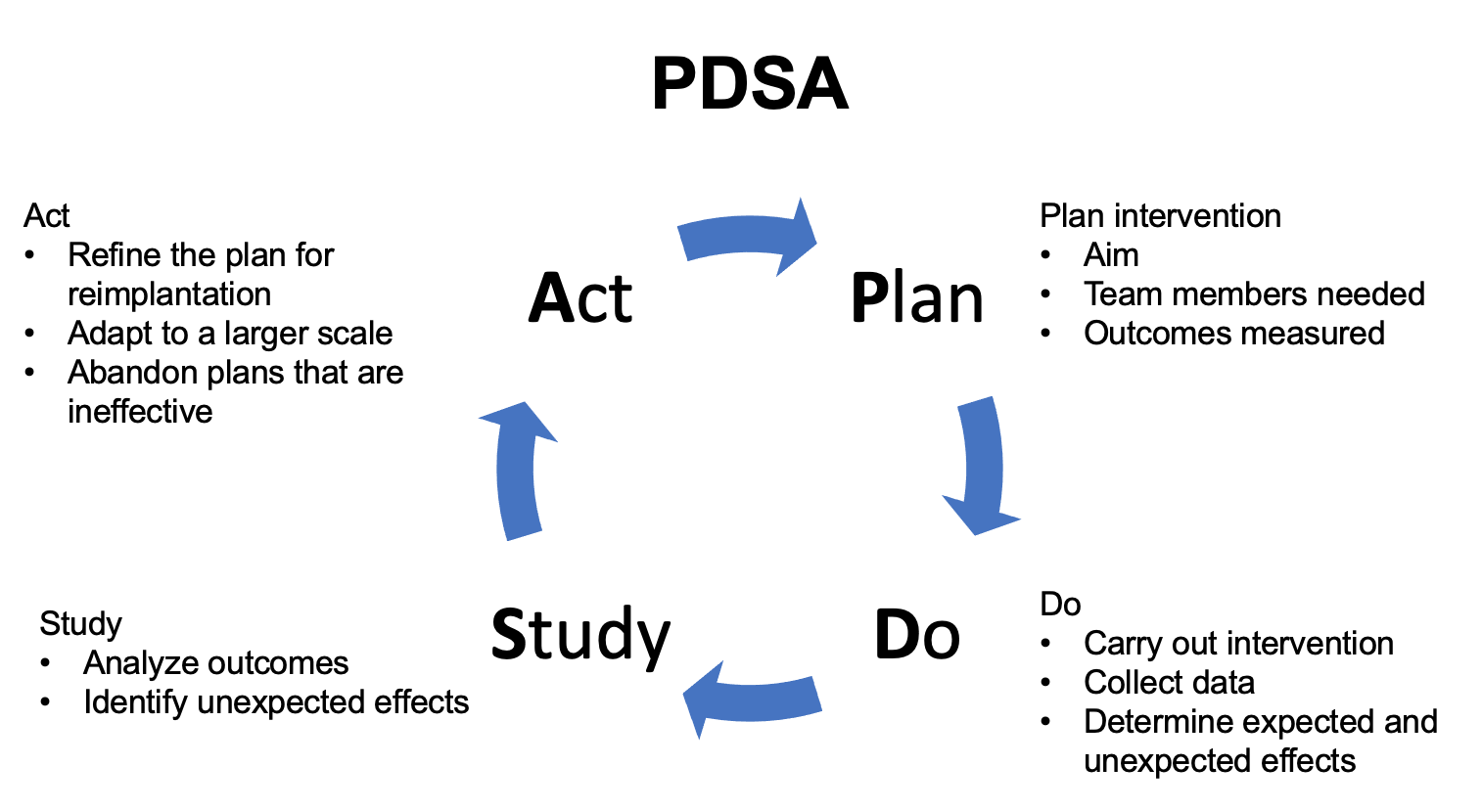
Figure 3 PDSA. Schematic detailing the Plan-Do-Study-Act (PDSA) approach.
Lean Six Sigma and DMAIC
Lean Six Sigma combines the approaches of Lean thinking and Six Sigma. Lean thinking originated from the Japanese automotive industry and is focused on reducing waste. With this approach, one first studies the process that requires improvement and reduces wasteful processes.38 Six Sigma is a structured approach to process and quality improvement that originated in manufacturing industries. The combination of the two approaches is known as Lean Six Sigma. Under this framework, teams approach QI projects through five phases: Define, Measure, Analyze, Improve, Control (Figure 4) In the Define phase, the issue to be addressed is identified. Baseline data is gathered during the measure phase. In the analyze phase, the root cause to the problem is deciphered and measures for improvement are implemented. In the control phase, implemented changes are maintained.39
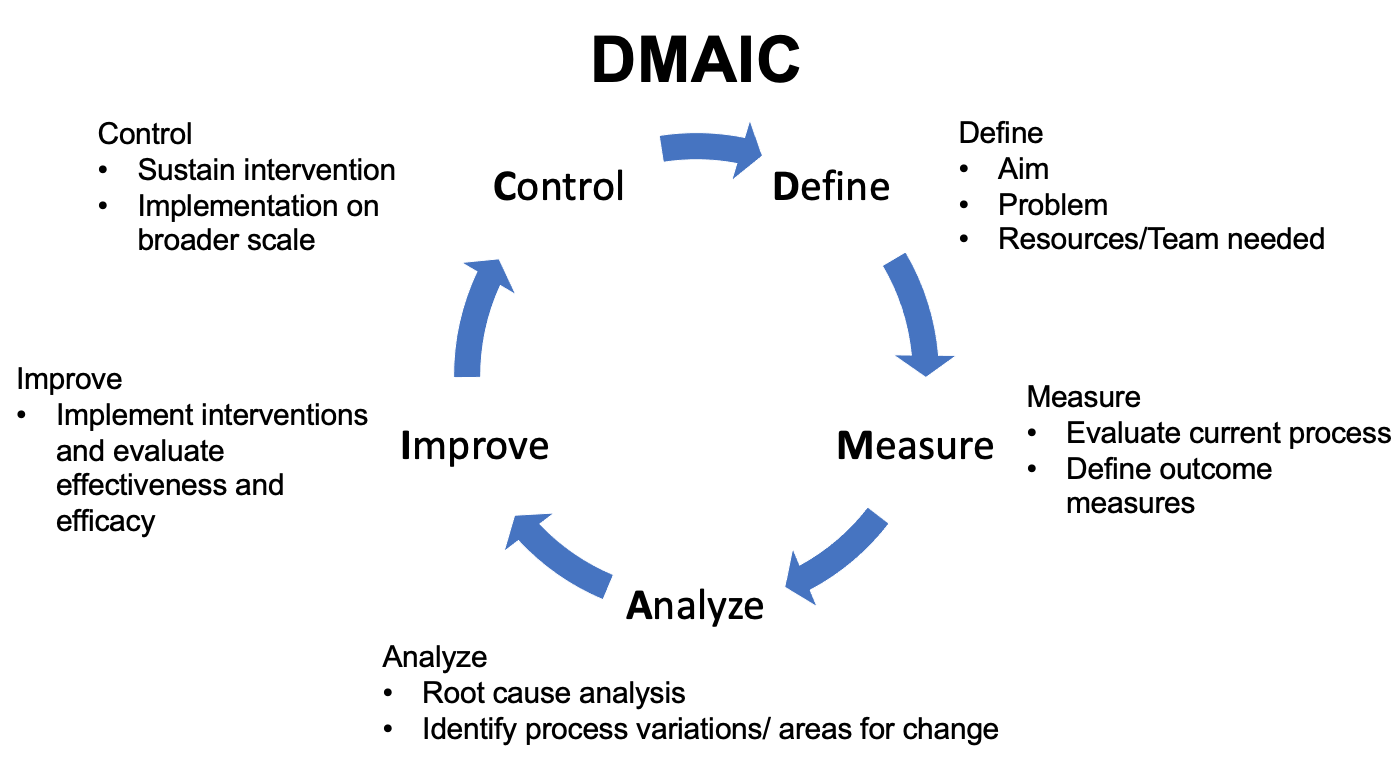
Figure 4 DMAIC. Schematic detailing the Define-Measure-Analyze-Improve-Control approach.
Future Directions
Quality improvement is gaining momentum in pediatric urology, but there is still a long way to go. Compared to standard clinical research, the methodology, implementation process, and management of global and institutional implementation barriers are especially critical in the success of QI projects. While journals including the Journal of Pediatric Urology are publishing more QI papers, these publications are still wanting in urologic literature. A discussion platform for QI enthusiasts in pediatric urology can help propel projects forward as we collectively navigate implementation barriers and learn from each other’s experiences and struggles. The relative rarity of pediatric urologic conditions also makes defining “quality” challenging as we are limited by the presence of high quality evidence to support best practices.36 However, implementation of data-driven practices may help better assess their efficacy and safety as we continue to challenge dogma. As pediatric urologists, our involvement and leadership in QI is critical as we strive for continuous improvement and move our field forward.
Suggested Readings
- Institute for Healthcare Improvement
- Hannick JH, O’Kelly F, Wolfstadt JI. Improving care in pediatric urology-A primer on quality improvement methodology and how to apply it to pediatric urology. J Pediatr Urol 2019; 15: 503, DOI: 10.1016/j.jpurol.2019.09.014.
- Cohen R. I.: Lean Methodology in Health Care. Chest 2018; 154: 1448, DOI: 10.1016/j.chest.2018.06.005.
- Koning H, Verver JP, Heuvel J. Lean six sigma in healthcare. J Healthc Qual 2006; 28: 4, DOI: 10.4018/978-1-4666-7320-5.ch009.
References
- Kohn LT, Corrigan JM, Washington MSD, editors. To Err is Human: Building a Safer Health System. 2000. DOI: 10.1016/s1051-0443(01)70072-3.
- Zhao LC, Lautz TB, Meeks JJ. Pediatric testicular torsion epidemiology using a national database: incidence, risk of orchiectomy and possible measures toward improving the quality of care. J Urol 2011; 186: 2009, DOI: 10.1016/j.juro.2011.07.024.
- Arevalo MK, Sheth KR, Menon VS. Straight to the Operating Room: An Emergent Surgery Track for Acute Testicular Torsion Transfers. J Pediatr 2018; 192: 178, DOI: 10.1016/j.jpeds.2017.09.009.
- Zee RS, Bayne CE, Gomella PT. Implementation of the accelerated care of torsion pathway: a quality improvement initiative for testicular torsion. J Pediatr Urol 2019; 15: 473, DOI: 10.1016/j.jpurol.2019.07.011.
- Heathcote S Sr., Duggan K, Rosbrugh J. Enhanced Recovery after Surgery (ERAS) Protocols Expanded over Multiple Service Lines Improves Patient Care and Hospital Cost. Am Surg 2019; 85: 1044, DOI: 10.1177/000313481908500951.
- Ljungqvist O, Scott M, Fearon K. C.: Enhanced Recovery After Surgery: A Review. JAMA Surg 2017; 152: 292, DOI: 10.1007/978-3-030-33443-7.
- Cain MP. Enhanced Recovery after Surgery Protocols in Pediatric Urology-How are we Doing and What Should we be Doing? J Urol 2018; 200: 952, DOI: 10.1016/j.juro.2018.08.037.
- Brindle ME, McDiarmid C, Short K. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations. World J Surg 2020; 44: 2482, DOI: 10.1007/s00268-020-05530-1.
- Short HL, Heiss KF, Burch K. Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2018; 53: 688, DOI: 10.1016/j.jpedsurg.2017.05.004.
- Chan YY, Rosoklija I, Meade P. Utilization of and barriers to enhanced recovery pathway implementation in pediatric urology. J Pediatr Urol 2021; 17: 294 1, DOI: 10.1016/j.jpurol.2021.01.044.
- Rove KO, Brockel MA, Saltzman AF. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol 2018; 14: 252 1, DOI: 10.1016/j.jpurol.2018.01.001.
- Chan YY, Chu DI, Hirsch J. Implementation and sustainability of an enhanced recovery pathway in pediatric bladder reconstruction: Flexibility, commitment, teamwork. J Pediatr Urol 2021. DOI: 10.1016/j.jpurol.2021.08.023.
- Rove KO, Strine AC, Wilcox DT. Design and development of the Pediatric Urology Recovery After Surgery Endeavor (PURSUE) multicentre pilot and exploratory study. BMJ Open 2020; 10: 039035, DOI: 10.1016/j.juro.2018.02.2313.
- Kelly BC, Vuolo M, Frizzell LC. Pediatric drug overdose mortality: contextual and policy effects for children under 12 years. Pediatr Res 2021. DOI: 10.1038/s41390-021-01567-7.
- Corona LE, Roth EB, Thao A. Opioid prescribing is excessive and variable after pediatric ambulatory urologic surgery. J Pediatr Urol 2021; 17: 259 1, DOI: 10.1016/j.jpurol.2021.01.008.
- Ahn JJ, Ellison JS, Merguerian P. A.: A Societies for Pediatric Urology survey of opioid prescribing practices after ambulatory pediatric urology procedures. J Pediatr Urol 2019; 15: 451, DOI: 10.1016/j.jpurol.2019.04.025.
- Garren BR, Lawrence MB, McNaull PP. Opioid-prescribing patterns, storage, handling, and disposal in postoperative pediatric urology patients. J Pediatr Urol 2019; 15: 260 1, DOI: 10.1016/j.jpurol.2019.02.009.
- O’Kelly F, Pokarowski M, DeCotiis KN. Structured opioid-free protocol following outpatient hypospadias repair - A prospective SQUIRE 2.0-compliant quality improvement initiative. J Pediatr Urol 2020; 16: 647 1, DOI: 10.1016/j.jpurol.2020.06.012.
- Mittal S, Shukla AR, Sahadev R. Reducing post-operative opioids in children undergoing outpatient urologic surgery: A quality improvement initiative. J Pediatr Urol 2020; 16: 846 1, DOI: 10.1016/j.jpurol.2020.09.022.
- Cardona-Grau D, Bush RA, Le HK. Reducing Opioid Prescriptions in Outpatient Pediatric Urological Surgery. J Urol 2019; 201: 1012, DOI: 10.1097/ju.0000000000000020.
- Villanueva J, Pifer B, Colaco M. A government mandated consent safely reduces opioid utilization for major pediatric genitourinary surgeries. J Pediatr Surg 2021. DOI: 10.1016/j.jpedsurg.2021.01.004.
- Skokan AJ, Kovell RC. Advances and Challenges in Transitional Urology: Caring for Adolescents and Young Adults with Lifelong Complex Genitourinary Conditions. Curr Urol Rep 2018; 19: 26, DOI: 10.1007/s11934-018-0774-3.
- Hsieh MH, Wood HM, Dicianno BE. Research Needs for Effective Transition in Lifelong Care of Congenital Genitourinary Conditions: A Workshop Sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. Urology 2017; 103: 261, DOI: 10.1016/j.urology.2016.12.052.
- Chu DI, Kayle M, Stern A. Longitudinal Trajectories of Clean Intermittent Catheterization Responsibility in Youths with Spina Bifida. J Urol 2021; 101097JU0000000000002204. DOI: 10.1097/ju.0000000000002204.
- Rague JT, Kim S, Hirsch JA. Assessment of Health Literacy and Self-reported Readiness for Transition to Adult Care Among Adolescents and Young Adults With Spina Bifida. JAMA Netw Open 2021; 4: 2127034, DOI: 10.1001/jamanetworkopen.2021.27034.
- Grimsby GM, Burgess R, Culver S. Barriers to transition in young adults with neurogenic bladder. J Pediatr Urol 2016; 12: 258 1, DOI: 10.1016/j.jpurol.2016.04.015.
- Duplisea JJ, Romao RL, MacLellan DL. Urological Follow-up in Adult Spina Bifida Patients: Is There an Ideal Interval? Urology 2016; 97: 269, DOI: 10.1016/j.urology.2016.06.025.
- Szymanski KM, Cain MP, Hardacker TJ. How successful is the transition to adult urology care in spina bifida? A single center 7-year experience. J Pediatr Urol 2017; 13: 40 1, DOI: 10.1016/j.jpurol.2016.09.020.
- Koyle MA, AlQarni N, Odeh R. Reduction and standardization of surgical instruments in pediatric inguinal hernia repair. J Pediatr Urol 2018; 14: 20, DOI: 10.1016/j.jpurol.2017.08.002.
- Nast K, Swords K. A.: Decreasing operating room costs via reduction of surgical instruments. J Pediatr Urol 2019; 15: 153 1, DOI: 10.1016/j.jpurol.2019.01.013.
- Hannick JH, O’Kelly F, Wolfstadt JI. Improving care in pediatric urology-A primer on quality improvement methodology and how to apply it to pediatric urology. J Pediatr Urol 2019; 15: 503, DOI: 10.1016/j.jpurol.2019.09.014.
- Buchhalter JR, Scantlebury MH, D’Alfonso S. Creation and implementation of an electronic health record note for quality improvement in pediatric epilepsy: Practical considerations and lessons learned. Epilepsia Open 2021; 6: 345, DOI: 10.1002/epi4.12480.
- Ellison JS. Society for Pediatric Urology (SPU): NSQIP update. Societies for Pediatric Urology, Virtual. 2011.
- Ogrinc G, Davies L, Goodman D. Squire 2.0 (Standards for Quality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. Am J Crit Care 2015; 24: 466, DOI: 10.1016/s1553-7250(15)41062-1.
- O’Kelly F, Hannick JH, Wolfstadt JI. Quality improvement in pediatric urology-a historical perspective on street pumps, puerperal fever, surgical infection, and contemporary methodology. J Pediatr Urol 2019; 15: 495, DOI: 10.1016/j.jpurol.2019.08.016.
- Langley GL, R. M, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed., San Francisco, California, USA: Jossey-Bass Publishers; 2009, DOI: 10.1080/10686967.1998.11919154.
- Cohen R. I.: Lean Methodology in Health Care. Chest 2018; 154: 1448, DOI: 10.1016/j.chest.2018.06.005.
- Koning H, Verver JP, Heuvel J. Lean six sigma in healthcare. J Healthc Qual 2006; 28: 4, DOI: 10.4018/978-1-4666-7320-5.ch009.
最近更新时间: 2023-02-21 20:03