56: Acute Scrotum
Este capítulo durará aproximadamente 27 minutos para leer.
Introduction
An “acute scrotum” refers to the rapid onset of a painful and/or swollen scrotum. Testicular torsion (TT) is the most worrisome diagnosis at the forefront of any clinician’s mind, and without early intervention, the testicle risks necrosis secondary to prolonged ischemia. Once TT is ruled out, other etiologies may be considered. Broadly speaking, the differential diagnosis of the pediatric acute scrotum also includes torsion of the appendix testis, epididymitis, orchitis, incarcerated hernia, vasculitis, testicular trauma, and cellulitis. In this chapter, we will highlight the evaluation of testicular torsion, torsion of the testis or epididymis adnexa, and epididymitis. The primary cause of these diagnoses is related to the anatomy of the testis or epididymis itself whereas the other diagnoses have secondary causes of scrotal swelling, pain, or infection (e.g., mumps orchitis secondary to Paramyxoviridae virus). Diagnosis and management of these other diagnoses in the differential require treatment of the primary cause and are discussed elsewhere in this textbook.
Anatomy and Embryology
The scrotum is a sac of skin and underlying tissue inferior to the phallus in which the testicles and epididymis are contained. Deep to the skin is dartos fascia, a continuation of Colles fascia of the perineum, dartos fascia of the penis, and Scarpa’s fascia of the abdomen. Dartos fascia is elastic in nature. It provides blood to the scrotal skin and, along with the cremasteric reflex of the testis, helps regulate testis temperature by contracting (elevating) or relaxing (dropping) the scrotum as a whole. The scrotum is divided into lateral compartments by the scrotal septum. Internally, the septum is composed of thickened dartos fascia. Externally the septum manifests as the median scrotal raphe.
The tunica vaginalis is the structural lining of the interior of the scrotum that surrounds the testicles. The tunica vaginalis is derived from the processus vaginalis (or vaginal process), an embryonic outpouching of the abdominal peritoneum that spans the length of the inguinal canal into the scrotum prior to its obliteration. After the inguinal portion is obliterated, the remaining distal sac surrounds the testis and consists of two layers: a visceral layer and a parietal layer. The visceral layer is adherent to the tunica albuginea, the outermost surface of the testis. The parietal layer reflects onto the inner surface of the scrotum.
The testis is an ovoid-shaped male gonad located within each side of the scrotum and which serves endocrine and reproductive functions via the production of hormones (namely, testosterone) and sperm. Sperm is produced within the seminiferous tubules of the testis and travels through the rete testis to the efferent ductules, which connect to the head of the epididymis. The epididymis is a tortuously coiled tube that rests upon the posterolateral aspect of the testis. It functions to store and mature sperm until release through the vas deferens en route to the ampulla of the vas, the reservoir for sperm mature for emission.
The spermatic cord is a major structure contained within the scrotum. It originates at the deep (internal) inguinal ring of the abdominal wall, runs through the inguinal canal, and passes through the superficial (external) inguinal ring into the scrotum. The spermatic cord contains nerves, vasculature, and ducts. It is surrounded by three layers of fascial tissue (superficial to deep): the external spermatic fascia (a continuation of the external oblique fascia), the cremaster muscle (continuation of the internal oblique musculature), and the internal spermatic spermatic fascia (continuation of the transversalis fascia. In addition to these individual layers, the spermatic cord includes (Figure 1):
Vasculature:
- Testicular artery (a branch of the aorta)
- Cremasteric artery and vein (a branch of the inferior epigastric vessels)
- Artery to the vas deferens (a branch of the superior vesical artery)
- Pampiniform plexus (venous network distal to the inguinal ring)
Nerves:
- Ilioinguinal nerve (joins the spermatic cord superficial to the external spermatic fascia from within the inguinal canal to provide sensation to the upper scrotum and penile skin)
- Genital branch of the genitofemoral nerve (supplies motor activity to the cremasteric muscle and sensation to the anterior scrotum)
- Autonomic nerves
Other:
- Vas deferens
- Lymphatic vessels
- Processus vaginalis
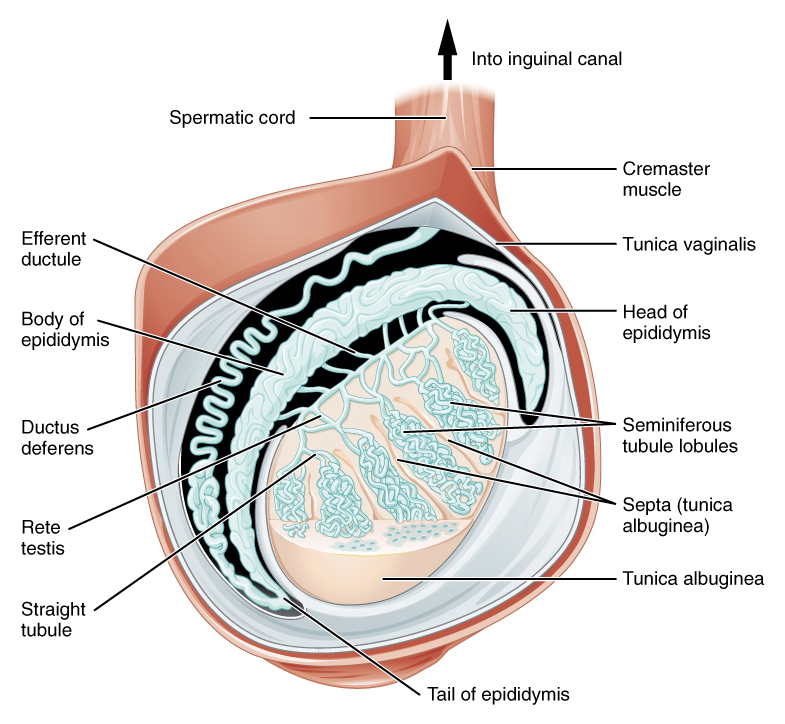
Figure 1 Distal contents of the spermatic cord and tunica vaginalis. Attribution: OpenStax College
The differentiation, development, and descent of the testes is described in other chapters.
General Evaluation and Diagnosis
For the pediatric patient presenting with acute testicular pain and/or scrotal swelling, a careful history and physical exam should be performed.
Key elements of a patient’s history should be obtained, including age, the nature of onset of pain (e.g., abrupt or gradual onset, presence of trauma), location and radiation of pain, and associated symptoms (e.g., abdominal pain, nausea or vomiting, hematuria, dysuria, fever, constipation). Associated symptoms can be helpful in both solidifying a diagnosis of TT or ruling out TT for other diagnoses. TT commonly presents with abdominal pain, nausea, and/or vomiting. On the other hand, dysuria and the presence of systemic fevers would suggest another etiology, such as epididymitis.
The physical exam should include an abdominal exam and genital exam. The abdominal exam should include assessment of costovertebral tenderness for evidence of flank pain, which may represent ureteric colic with referred pain to the groin. Evidence of hernia or cellulitis in the groin region should also be noted.1 The presence of isolated abdominal pain should still prompt a genital exam to rule out an acute scrotum, especially in younger boys who cannot adequately communicate their needs, boys with developmental delay, and boys with potentially altered sensorium.2
The genital examination includes assessment of the scrotum and bilateral testes, beginning with inspection and followed by palpation. Inspection of the skin should note erythema suggestive of cellulitis. In lighter-skinned children, a faint blue-ish hue (“blue dot” sign) may be seen on the lateral scrotal skin in children with appendix testis or epididymis torsion, though this physical exam sign is present in a minority of cases and not relevant to children with darker skin.3 Gentle palpation may be achieved by rolling the entire testicular surface between the thumb and forefingers. This exam is often difficult to perform in the setting of TT due to testicular edema and the associated reactive, tight hydrocoele. A high-riding testis when compared to the contralateral side should be noted and is suggestive of TT (by way of elevation of the testis as the spermatic cord twists and shortens). The lie of the testis, whether vertical or horizontal, is also of interest. Importantly, the bell clapper deformity results in a horizontal lie and is present in males with an abnormally superior attachment of the tunica vaginalis to the spermatic cord. This anatomical variant permits free rotation of the testis and predisposes the patient to intravaginal torsion (discussed later). Note this abnormality is difficult to distinguish in a normal setting and is unlikely to be established in the setting of TT secondary to edema and skin changes.
Additional examination maneuvers may elicit the diagnosis:
- Scrotal masses should be defined by transillumination (indicative of a hydrocele) and attempted reduction (indicative of a patent processus vaginalis)
- The cremasteric reflex should be assessed by stroking the ipsilateral thigh of the affected testicle. The reflex normally causes cremasteric contraction and elevation of the testis within the scrotum. Absence of the reflex in a patient presenting with acute scrotal pain classically suggests the diagnosis of TT; this finding is 100% sensitive and 66% specific.4 However, in a young child, the reflex arc may be underdeveloped and absent in an otherwise healthy individual.5,6 Therefore, it is important to first assess for a cremasteric reflex on the contralateral “normal” side to establish a baseline exam.
- Elevating the scrotal contents is a maneuver taught to distinguish between epididymitis and TT. The relief of pain upon scrotal elevation, classically called a positive Prehn’s sign, is suggestive of epididymitis and is not reproducible in TT, though this is not always reliable test in children.1
Initial diagnostic testing, which will be further discussed, usually includes:
- Urinalysis (UA) and urine culture. These tests are performed to rule out urinary tract infection and bacterial epididymitis. Children presenting with TT will not generally have signs of infection on properly-collected urine specimens
- Color Doppler Ultrasonography (CDUS). This is a confirmatory test identifying lack of vascular flow to a testis
Testicular Torsion
Epidemiology
The reported incidence of TT is as high as 1 in 4,000 for males age 25 or younger7 to as low as 3.8 per 100,000 in males age 18 or younger.8 Incidence has a bimodal distribution, peaking in the first year of life (typically first month) and the pubertal period (12 years of age). This bimodal distribution corresponds to the difference in pathogenesis in the newborn and peripubertal period (extravaginal torsion versus intravaginal torsion of the spermatic cord, respectively).
Pathogenesis
TT occurs when the testicle rotates around its spermatic cord. The twisted cord compresses the testicle’s venous network, causing testicular edema, restriction of venous outflow, and later occlusion of arterial inflow. Prolonged ischemia is deleterious and if left unresolved minimizes the chance of testicular viability.
Two types of TT exist: intravaginal and extravaginal torsion.
Extravaginal torsion occurs when the tunica vaginalis and its contents, including the testis, turn as a unit about the spermatic cord axis. This phenomenon is made possible by unfused scrotal layers in the neonatal period, allowing the tunica vaginalis to move within the dartos fascia-lined hemiscrotal compartment. Extravaginal torsion occurs exclusively in the neonatal period or prenatally and has been reported to occur as late as 1–3 months after birth.9 Neonatal TT is rare compared to intravaginal torsion with an incidence of 6.1 per 100,000.10
Intravaginal torsion occurs when the testicle twists about the spermatic cord within the tunica vaginalis. The so-called “bell clapper deformity” is the primary risk factor predisposing to intravaginal torsion. Normally the visceral and parietal tunica vaginalis layers are fused posteriorly and inferiorly, securing the testis within the tunica vaginalis compartment. In males with a bell clapper deformity, the tunica vaginalis completely invests the testis, epididymis, and distal length of the spermatic cord without visceral and parietal fusion points.11 An autopsy series reported a 12% incidence of the bell clapper deformity, which, notably, is higher than the incidence of TT.12 The high incidence of bilateral bell clapper deformity rationalizes preventative orchidopexy on the contralateral, unaffected side.
It should be noted that perinatal (extravaginal) torsion is not thought to be associated with a contralateral bell clapper deformity and subsequent risk for intravaginal TT. Conversely, intravaginal torsion on one side likely signals the presence of a contralateral bell clapper deformity and risk for subsequent, metachronous contralateral intravaginal torsion. In a single-surgeon series of 50 consecutive patients taken to the operating room for exploration of a nonpalpable testis revealing a testicular nubbin, only 1 patient showed evidence of a contralateral bell clapper deformity. The authors contrasted this with 27 straight cases of TT in older boys in whom 21 (78%) had a contralateral bell clapper deformity.13
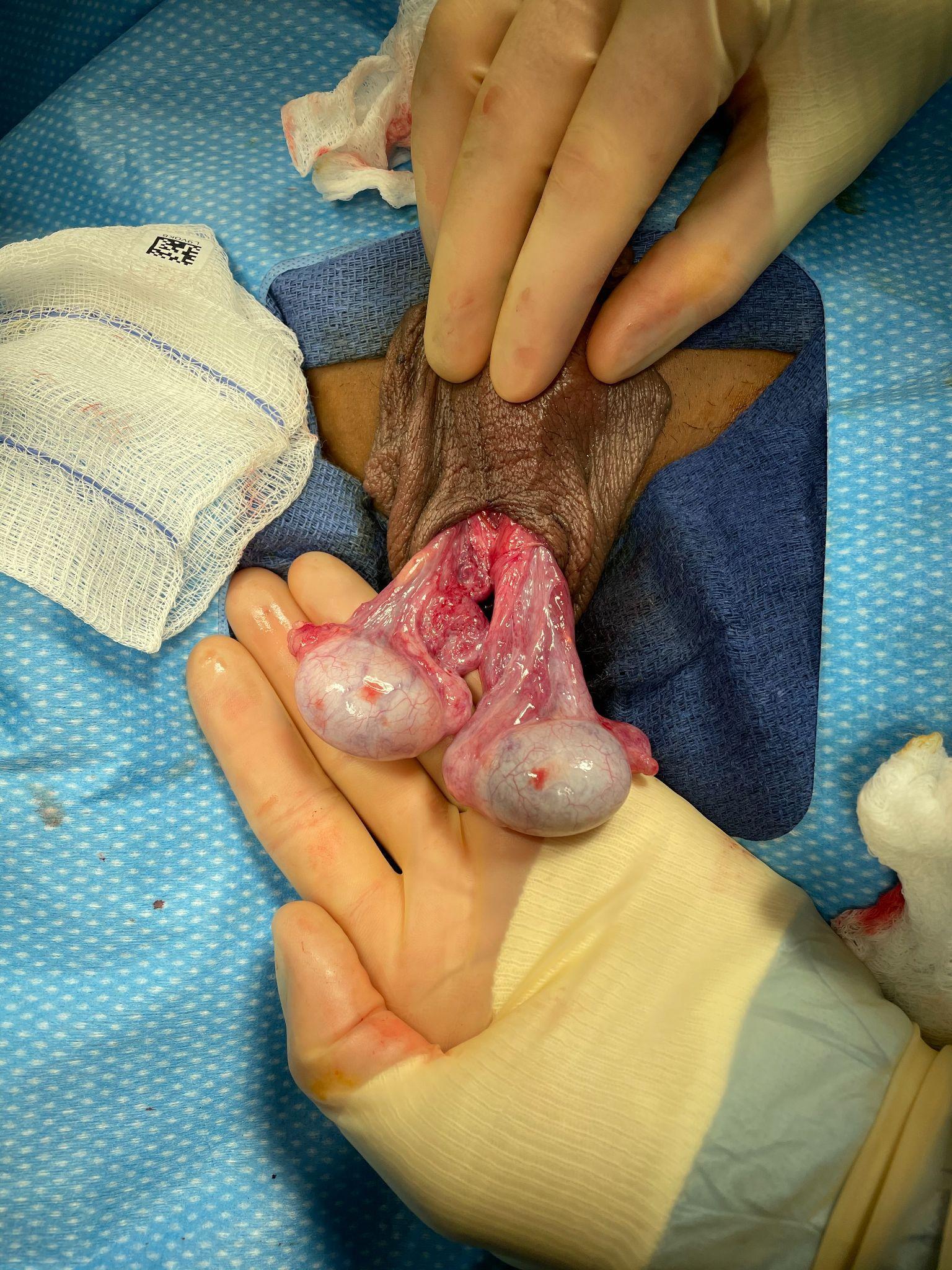
Figure 2 Bell clapper deformity in a case of intravaginal torsion (the left testis has undergone detorsion). Neither testis possess fusion of the tunica vaginalis visceral and parietal layers, and as a result, the tunica vaginalis has retracted high on the spermatic cord and both testes are left to hang freely, demonstrating a transverse lie relative to the spermatic cord. One can imagine if the tunica vaginalis parietal layer were intact that the testes would be hanging completely within the tunica vaginalis, like the clapper of a bell.
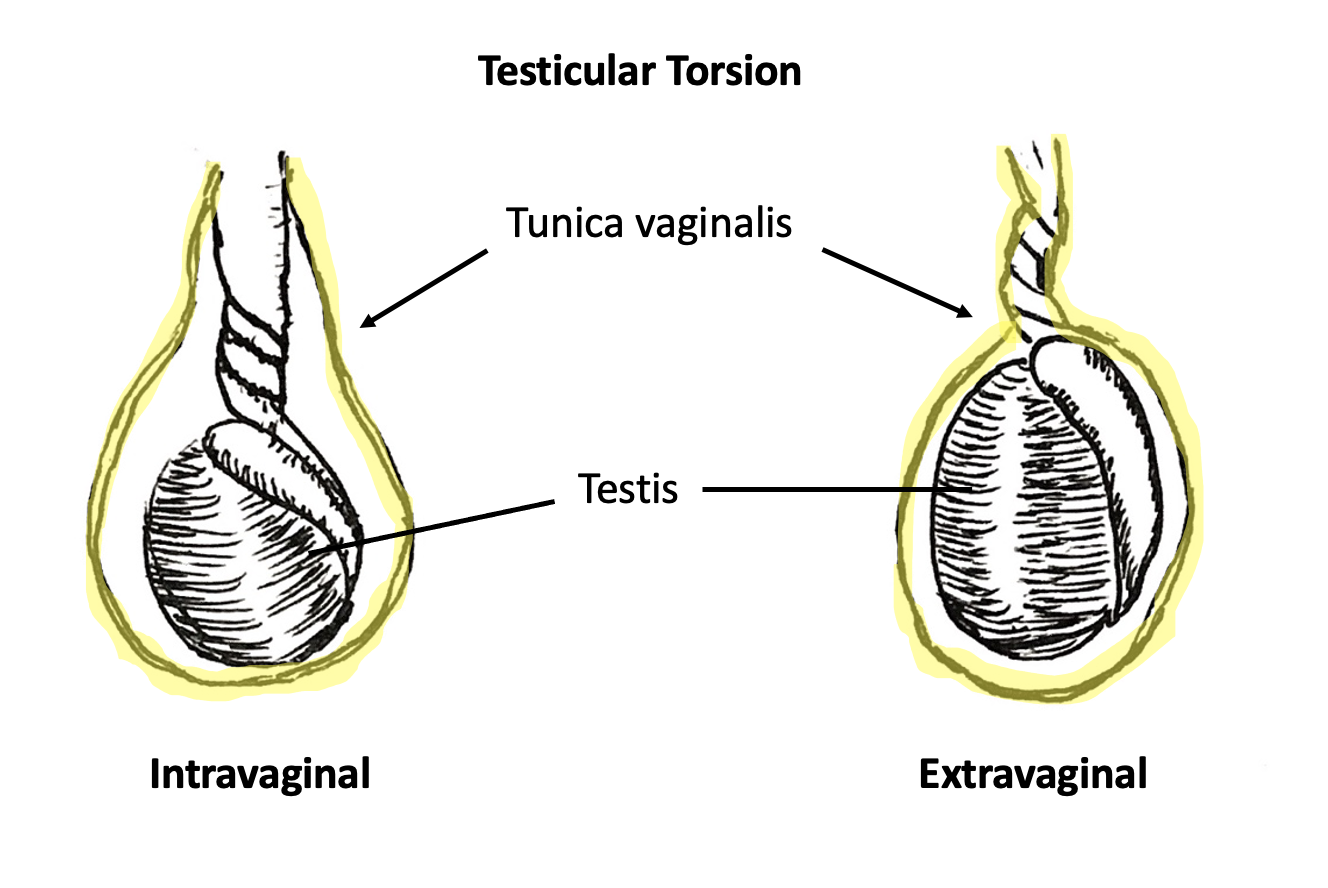
Figure 3 Intravaginal torsion (left) and extravaginal torsion (right). Tunica vaginalis is highlighted in yellow. In intravaginal torsion, spermatic cord twisting occurs separately and within the tunica vaginalis. In extravaginal torsion, the spermatic cord and tunica vaginalis twist as a singular unit.
Evaluation and Diagnosis of Testicular Torsion in the Neonate
The presentation of a neonate with extravaginal TT is variable, which is likely reflective of the disease process as well as the circumstances surrounding the birthing and early care of a neonate. Newborn babies may collect edema in the perineum during the birthing process, and the scrotum can appear particularly enlarged and erythematous in the early hours after birth. There may also be an effect from maternal hormones. Congenital hydroceles are common and may be largest immediately after birth. Collectively, these changes may make it difficult for clinicians to differentiate specific epididymal and testicular anatomy on palpation alone.
Extravaginal TT in a neonate will commonly present with an enlarged, hardened hemiscrotum. Birthing and postnatal care teams need to be keen to subtle exam differences between the respective hemiscrotum. The right and left hemiscrotum should be inspected and palpated individually, not as a unit. Increased firmness to one side, loss of scrotal rugae, or differential skin changes should be noted and trigger the need for repeated exams. Remarkable findings on initial exam or changes on serial exams should prompt urgent urological consultation.
Extravaginal torsion noted at the time of birth is unlikely to be salvageable. In a review of the literature, over 90% of neonatal torsions considered either “prenatal” or “postnatal” underwent unilateral orchiectomy.14 More important than salvaging the more obviously affected side is timely diagnosis or prevention of asynchronous extravaginal torsion. This frequently requires surgical exploration as unilateral scrotal exam findings may be so profound that they obscure findings of the contralateral side. Additionally, CDUS is technically difficult in a neonate, particularly at centers where sonographers are not accustomed to imaging newborns. Urgent exploration of the neonatal scrotum in settings of suspected unilateral neonatal extravaginal torsion is controversial. The decision to surgically explore the scrotum of a newborn boy in the setting of a unilateral exam suggestive of extravaginal torsion pits the unlikely salvageability of the affected testis and anesthetic risks to the newborn against the rare but devastating consequences of asynchronous extravaginal TT. Case reports of bilateral, asynchronous extravaginal torsion abound in the literature. Recent publications have tried to quantify the risk of asynchronous torsion to the neonate. One review of the literature encompassing 152 studies representing 1336 patients reported 11.8% of all neonatal torsion events were asynchronous with a median time to second torsion of 1 day. The authors calculated a number needed to treat (e.g. surgically explore) to avoid bilateral orchiectomy to be 1.6, and the number needed to treat to avoid solitary testicular atrophy to be 2.6. A separate 20-year, single-institution chart review reported a 3% risk of asynchronous bilateral perinatal torsion.15 Given the improved safety of perinatal anesthesia in recent decades, it is the authors’ opinion that the benefits of early and urgent surgical exploration should be considered in the setting of unilateral perinatal torsion.
Evaluation and Diagnosis in the Older Child
For any patient presenting with acute onset scrotal pain, a diagnosis of TT should be promptly confirmed or ruled out. It is the diagnosis that poses the most time-sensitive threat to the organ. Clinicians should bear in mind a focused history and physical exam are the only prerequisites for surgical exploration. CDUS may confirm the suspected diagnosis of TT or rule out end-organ ischemia. The presence of vascular flow on CDUS does not rule out the presence of developing ischemia (as venous outflow is likely to be reduced prior to edema and vascular congestion that limits arterial inflow), recent or intermittent TT, or incomplete or partial torsion. In the latter setting, the spermatic cord may rotate about its axis enough to cause vascular compromise but not completely enough to cause abrupt cessation of blood flow.
History
The classic presentation of intravaginal TT consists of acute onset testicular pain in a peripubescent male. Patients or their parents often describe sudden onset of severe pain. The pain may begin at rest, and patients will commonly report waking from sleep with sudden pain. Patients may report pain following trauma to the scrotum. In this latter setting, it's hypothesized the trauma itself is unlikely the cause of TT but a misremembered or unrelated event. In fact, history of recent genital trauma has been identified as a cause of misdiagnosis and resultant delay in treatment.2
Common associated symptoms may include abdominal pain, nausea, and vomiting.16 Importantly, abdominal pain and/or nausea with or without emesis may precede the onset of testicular pain. This creates a diagnostic enigma with potentially detrimental consequences to clinicians who hone their exam to the abdomen and omit a genitourinary exam. Isolated abdominal pain has been shown to be significantly associated with delayed presentations (>24 hours) and higher rates of orchiectomy.2
The presence of associated symptoms may be helpful in prioritizing differential diagnoses as appendix testis or epididymis torsion and epididymitis or orchitis are more likely to present as isolated scrotal pain. Patients uncommonly report dysuria or gradual onset of pain. These symptoms should prompt clinicians to consider other diagnoses, such as epididymitis (sterile or bacterial), orchitis, or urinary tract infection.
Physical Exam
Abdominal and genitourinary exam should be performed. Scrotal skin may reveal erythema, induration, or warmth. A tight hydrocele with decreased presence of scrotal rugae is often noted. In those with fair skin presenting in a delayed setting, a tight hydrocele with a dusky hue may reflect a necrotic testis and/or hematocele. Such skin changes have been shown to be predictive of TT.17
An abnormal cremasteric reflex suggests an increased likelihood of TT.17,18 However, the presence of a normal cremasteric reflex cannot sufficiently rule out a diagnosis of TT.19 Likewise, the absence of a cremasteric reflex is not highly specific for the diagnosis. When checking for cremasteric reflexes, providers should first examine the contralateral side as the absence of an ipsilateral reflex is only clinically relevant in the presence of a contralateral reflex. Clinicians may find the affected testis has a horizontal lie (bell-clapper deformity) or a “high-riding” position (from the twisted spermatic cord elevating the testis into the inguinal canal).
Utilization of the TWIST score (introduced in 2013 by Barbosa et al) may allow clinicians to risk-stratify patients into low-, intermediate-, and high-risk for TT based on physical exam alone. A summed score of 0-7 is calculated after determining the presence of 5 symptoms: hard testis (2 points), testis swelling,2 absent cremasteric reflex,1 nausea/vomiting,1 and high riding testis.120 According to the score algorithm, high risk patients (a score ≥5) have a positive predictive value of 100% for TT, thus clinicians can consider proceeding directly to surgical exploration as there is minimal benefit to CDUS. Low risk patients (≤2) have a negative predictive value of 100% for TT, thus they do not require CDUS to rule out torsion. Intermediate risk patients may benefit from CDUS to confirm the diagnosis. A study evaluating the usefulness of the TWIST score amongst nonurological nonphysician providers determined a PPV of 93.5% and NPV of 100% when high risk was defined as a score of greater than 6, intermediate risk as 1-5, and low risk as 0.21 (Figure 4)
Table 1 The TWIST score and risk stratification groups. Adopted from Barbosa 2013
| Symptom | Points Awarded |
|---|---|
| Hard Testis | 2 |
| Testicular Swelling | 2 |
| Absent Cremasteric Reflex | 1 |
| High-riding Testis | 1 |
| Nausea and/or Vomiting | 1 |
| Total | |
| High risk | ≥5 |
| Intermediate risk | 3-4 |
| Low risk | ≤2 |
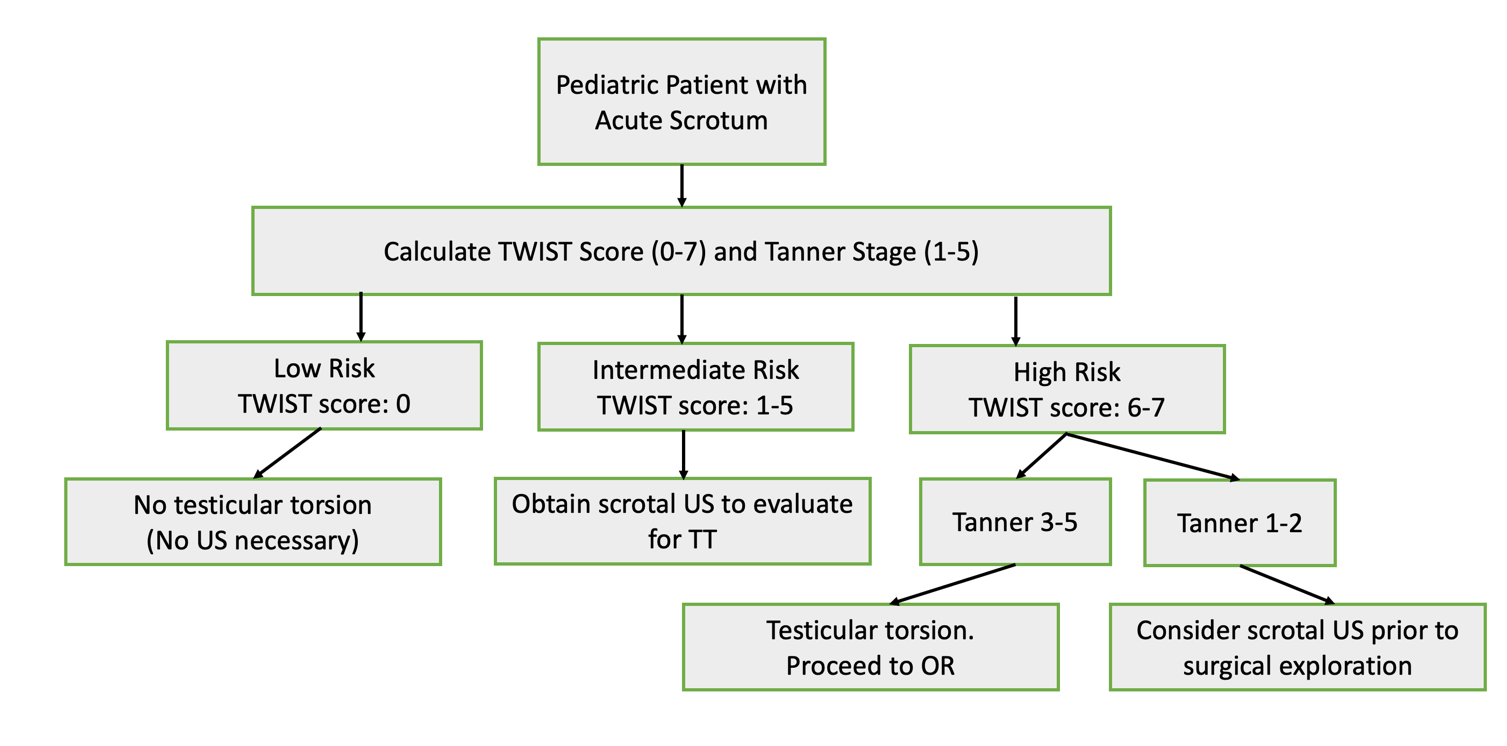
Figure 4 Flow diagram with proposed management of testicular torsion, adapted from Sheth 2016. Notably, this study evaluated the usefulness of the TWIST score amongst nonurologic nonphysician providers (to mirror emergency room triage personnel) and uses different thresholds for low, intermediate, and high-risk scores than the original Barbosa et al. 2013 description
Initial Tests
Urinalysis (UA) is a low-cost, rapid test useful in the setting of acute scrotal pain. It can be helpful to distinguish TT from bacterial epididymitis or urinary tract infection, both of which the UA would be expected to demonstrate pyuria. In TT, the UA would be expected to be unremarkable.
Imaging
Routine use is controversial, and studies assessing the accuracy and utility of imaging abound.22,23,24 If the diagnosis of TT is equivocal based upon the gathered history and physical, then imaging may be pursued. However, imaging should not be used as a substitute for an adequate history and physical exam. Unnecessary imaging can delay surgery and increase cost of care. Imaging is not required to diagnose TT.
Color Doppler Ultrasonography
CDUS is useful for visualization of testicular anatomy, including spermatic cord architecture, testicular blood flow, and testicular echotexture. Decreased or absent testicular blood flow on US has a specificity of 99% and a sensitivity of 63% for TT.24 As a rule of thumb, imaging should always be compared to the contralateral, unaffected side. In TT, the affected testis may appear edematous and enlarged. Clinicians should pay particular attention to comparative testis volumes in cases of equivocal CDUS as this can be a clue to the diagnosis. A reactive hydrocele may also be present.25 Heterogenous echotexture in the setting of absent Doppler signal likely indicates the onset of coagulative necrosis of a nonviable testis.
Occasionally, normal or increased arterial flow is visualized in a patient with TT, generating a misleading false-negative result. One multi-center study demonstrated 24% of their confirmed spermatic cord torsions had normal or increased testicular flow on preoperative CDUS.26 Conceptually, it has been postulated that as the spermatic cord twists, venous outflow from the testis is likely affected prior to arterial inflow. Therefore, decreased or maintained arterial flow may hark the onset of testicular ischemia and should not necessarily be regarded as reassuring in the setting of absence or decreased outflow. Decreased blood flow may be suggestive of partial or incomplete torsion and intermittent torsion of the spermatic cord. It should be highlighted that Doppler and vascular waveform measurement is highly dependent on the sonographer and substantially more difficult to obtain in babies and young children. In settings of discordant history, physical exam, and CDUS findings, clinicians should exercise a high index of suspicion for partial or intermittent TT.
High Resolution Ultrasonography
high resolution ultrasonography (HRUS) is a more sensitive (97.3%) and specific (99%) imaging method compared to CDUS.26 It allows for direct visualization of a torsed spermatic cord. Studies have shown well-trained sonographers and radiologists can accurately identify tortuosity in the spermatic cord via the so-called “snail-shaped” or “whirlpool” spermatic cord signs. There is controversy in the literature about the utility of the HRUS identification of spermatic cord “whirlpool” signs. When correctly diagnosed in the setting of abnormal testis blood flow, these spermatic cord abnormalities can be definitive evidence of ongoing intravaginal torsion. However, a common criticism of HRUS-identified spermatic cord abnormalities is that literature highlighting these abnormalities have been unsuccessful in identifying the denominator of cases or how many children with torsion do not have identifiable spermatic cord changes on TT. In other words, the absence of identified or reported signs of spermatic cord tortuosity should not rule out the diagnosis of TT, and clinicians should not use HRUS-findings of the spermatic cord alone as indication for exploration.
Nuclear Scintigraphy
This test uses intravenous technetium-99m pertechnetate to evaluate testicular perfusion. Prior to CDUS, scintigraphy was the imaging test of choice for suspected TT. Studies have reported similar sensitivity and specificity to CDUS.27,28 However, the scan can take several hours to perform, is not always readily available, and risks prolonged testicular ischemia time. For these reasons, scintigraphy is rarely utilized in the modern diagnosis of TT.
Treatment Options
TT requires prompt surgical correction. Both the time since onset of pain (conceptualized as being equivalent to testicular ischemia time) and degree of spermatic cord torsion directly impact testis salvage rates.29,30 As a rule of thumb, the testis is most likely to be viable (>90% likelihood) if scrotal exploration is performed within the first 6 hours of symptom onset. After the initial 6-hour window, the testicle may still be viable but at precipitously decreasing rates, thus highlighting the importance of timely intervention.31 However, even within this timeframe, patients may experience some degree of postoperative testicular atrophy from ischemia damage.
Manual Detorsion
Manual detorsion, or bedside rotation of the testis within the scrotum, is an option for initial management while awaiting urologic consultation and emergent surgical treatment or transfer to another facility. The maneuver can restore some blood flow to the testis and improve odds of testicle viability at time of exploration.32,33 Manual detorsion does not obviate the need for surgery and should not delay surgical intervention.
The classic teaching in TT manual detorsion is that the testis twists about the spermatic cord in a lateral to medial direction. Steps to achieve manual detorsion instruct the clinician to unwind the spermatic cord in the opposite direction: medial to lateral. The motion of rotating the testicle away from midline is what is commonly referred to as “opening the book.” The clinician should grasp the affected testis between his or her thumb and forefinger and gently rotate the testis within the scrotum outward toward the thigh for a full 360 degrees. Successful manual detorsion is usually suggested by immediate relief of pain and return of arterial flow on CDUS. Multiple rotations may be necessary depending on how many twists the cord possesses. In an ideal scenario, point-of-care ultrasound or CDUS can be used to objectively demonstrate improvement in blood flow. If pain relief is not achieved or is worsened by manually detorsing the testicle away from midline, detorsion through an inward (medial) rotation could be attempted.
Contrary to this classic teaching, retrospective studies demonstrated that at least a third of their cases of TT occurred in the lateral direction.29,34 Therefore, while the “opening the book” technique is likely to reduce torsion of the spermatic cord and potentially restore blood flow in the majority of cases, clinicians who attempt manual detorsion need to be attentive to the possibility of either incomplete detorsion of a spermatic cord with several revolutions or increasing the degrees of revolution, the latter of which may worsen the degree of ischemia. Therefore, it is the authors’ opinion manual detorsion ought not to be attempted if surgical intervention is readily available. Additionally, manual detorsion ought not be attempted if the diagnosis is in question or if the maneuver will delay definitive surgical intervention. If clinicians performing manual detorsion are not those who will provide definitive surgical intervention, it is vital to communicate the maneuver was attempted and the result.
Surgical Correction
Surgery management entails either detorsion and orchidopexy (fixation of the testicle to scrotal dependent wall) or orchiectomy of the affected testis, depending on the viability of the organ when examined intraoperatively. In either case, orchidopexy of the contralateral testis is the standard of care given the high probability of a contralateral bell clapper deformity.
Overview of Surgical Technique:
A midline raphe incision or bilateral horizontal incisions are most common. A midline raphe incision allows for each hemi scrotum to be explored through a single incision.
- Midline scrotal incision
- Electrosurgery dissection through dartos and into affected hemiscrotum
- Delivery of tunica vaginalis through dartos incision
- (Inspection of tunica vaginalis and spermatic cord for extravaginal torsion in cases of neonatal torsion)
- Incision and eversion of tunica vaginalis to allow delivery and inspection of affected testicle and epididymis. Note the presence of intravaginal torsion of the spermatic cord and color of the testis and epididymis
- Detorsion of spermatic cord and testis, noting direction and degrees of torsion
- Envelopment of detorsed testis in a warm gauze sponge
- Perform same electrosurgery dissection of dartos on the contralateral hemiscrotum to expose contralateral tunica vaginalis and testicle
- Incision and eversion of tunica vaginalis to allow delivery and inspection of contralateral testicle and epididymis. Note the presence of a bell clapper deformity
- Return to detorsed testis and repeat inspection to determine viability. If obviously nonviable, perform an orchiectomy. If viable, perform orchidopexy. This is a subjective assessment and often variable between providers. If viability appears indeterminate, providers may choose to perform micro-Doppler assessment of the testis to search for arterial flow or incise the testis with an equatorial incision to identify bleeding and healthy-appearing seminiferous tubules secondary to release of compartment syndrome pressure. In the case where an equatorial tunica vaginalis fasciotomy leads to tunica vaginalis bleeding, healthy-appearing seminiferous tubules, and overall improvement in testis color, tunica vaginalis patch augmentation of the tunica albuginea has been described.35 (Video 1)
- An affected testis that has been determined viable and the normal contralateral testis should be secured in the scrotum to prevent future torsion. Testes ought to be secured with 3 points of fixation, either to each other (through the scrotal septum as in a “septopexy” technique) or in a dependent position in their respective hemiscrotum. The authors use a 5-0 polypropylene (non-absorbable) suture
- Close the scrotal incision(s) in a typical fashion. For cases where an orchiectomy is performed in the setting of long-standing, delayed-presentation of testis torsion with substantial edema, a scrotal drain may be left to help prevent infection, hematocele, or hydrocele formation
[Video 1](#video-1){:.video-link}. Intraoperative management of testicular torsion. In this case, surgical correction included provision of a tunica vaginalis flap. Note: This video was created for and featured in the American Urological Association core curriculum educational video library.
Suggested Follow-Up
Patients who undergo surgical management for testicular torsion should be seen at least once in outpatient clinic follow-up to verify proper wound healing and a non-concerning physical exam. After attempted testicular detorsion and orchidopexy for TT, clinicians should document the size of testes and the presence or absence of atrophy to an affected testis. The term “testicular salvage” is best reserved for affected testes which have shown preserved volume in follow-up.
During follow-up, the authors typically discuss the following topics with patients and their families:
- In the setting of orchidopexy of an affected testis, the appearance of and vascular flow to the affected testis should the patient ever receive another CDUS for another reason
- In the setting of orchiectomy of an affected testis, there is potential for a testicular prosthesis after completion of puberty
- Although recurrent testicular torsion is theoretically impossible, patients may feel pain or abnormalities for other reasons. Patients may benefit from monthly self-exams and should alert their parents or doctors immediately if they feel pain or palpate something different
- All patients, especially those who have undergone orchiectomy for TT and now have a solitary testis, should be encouraged to wear a protective athletic cup during potentially contact sports and recreation. There are modern, slim-profile athletic cups that fit into comfortable compression shorts and sliding pants
Torsion of the Testis Adnexa
Both the testis and epididymis commonly have a small, associated appendage off their superior pole. The appendix testis is an embryologic remnant of the paramesonephric duct. The appendix epididymis is an embryologic remnant of the mesonephric duct system. Either of these structures can twist about their axis, much in the same way as the spermatic cord twists in intravaginal TT, leading to ischemia and eventual necrosis. Presentation can mimic TT with abrupt onset of scrotal pain and swelling. There are, however, some key pertinent negative findings on history and physical exam the authors have anecdotally noticed in comparing testis adnexal torsion with TT:
- Patients with adnexal torsion are less likely to report nausea and vomiting. This is likely secondary to the lack of visceral sensation from adnexal ischemia compared to testis ischemia
- Patients with adnexal torsion are not likely to have an elevated testis lie within the scrotum
- Patients with adnexal torsion often have a good testicular flow on CDUS with characteristic findings of a heterogeneous, avascular small structure adjacent and separate from the testis36 (See Figure 5 for a representative image from the authors’ collection)
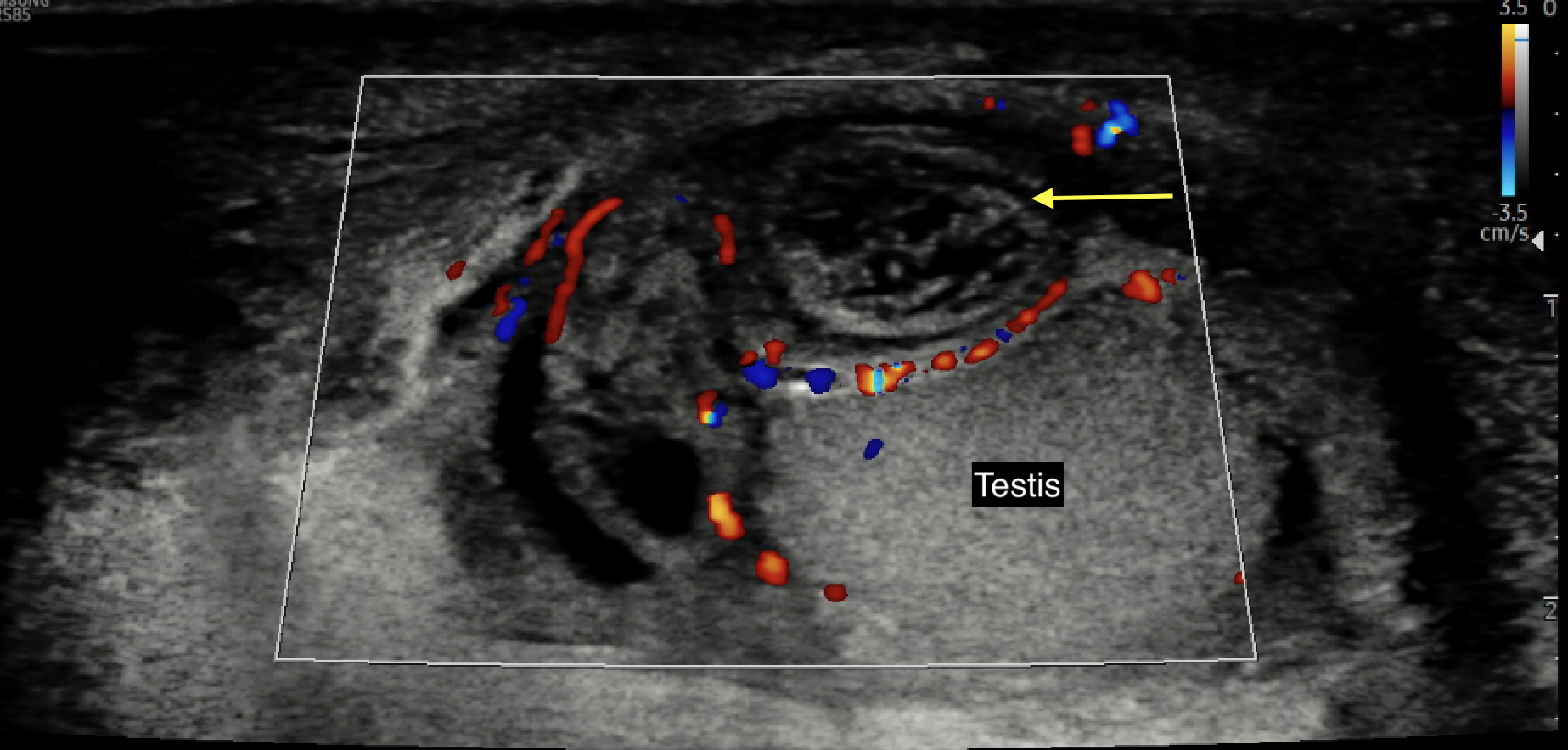
Figure 5 Color Doppler ultrasound image of an avascular, edematous, and heterogeneous-appearing torsed appendix testis or epididymis (yellow arrow)
On physical exam, adnexal torsion will present with a swollen, tender hemiscrotum. The testis will appear to have a normal, dependent lie. In light-skinned individuals, an occasional “blue dot” sign may be identified on the lateral scrotal wall, corresponding to the appearance of the ischemic appendage through scrotal skin thinned by a translucent hydrocele sac. To patients who can tolerate a thorough exam, this point will correspond to focal tenderness. Note that the blue dot sign has been reported in as few as 10% of adnexal torsion cases and will be difficult to identify in dark-skinned patients.
If the diagnosis is in question or testicular ischemia cannot be ruled out, surgical exploration is recommended. At exploration, the surgeon will find a torsed appendix with an otherwise normal appearing testis and epididymis (Figure 6 and Figure 7). The adnexal appendage can be surgically excised and the scrotum closed. For cases where good testicular flow is confirmed, history and objective data are inconsistent with TT, and adnexal torsion is likely, surgical exploration can be deferred.
Treatment for adnexal torsion is conservative and includes rest, periodic ice in the acute period, scrotal elevation, and non-steroidal anti-inflammatory medications. The authors have routinely ordered a follow-up scrotal US within one week if pain persists to confirm the diagnosis and 4-6 weeks after pain resolution to document the adnexal mass seen on initial scrotal US has regressed.
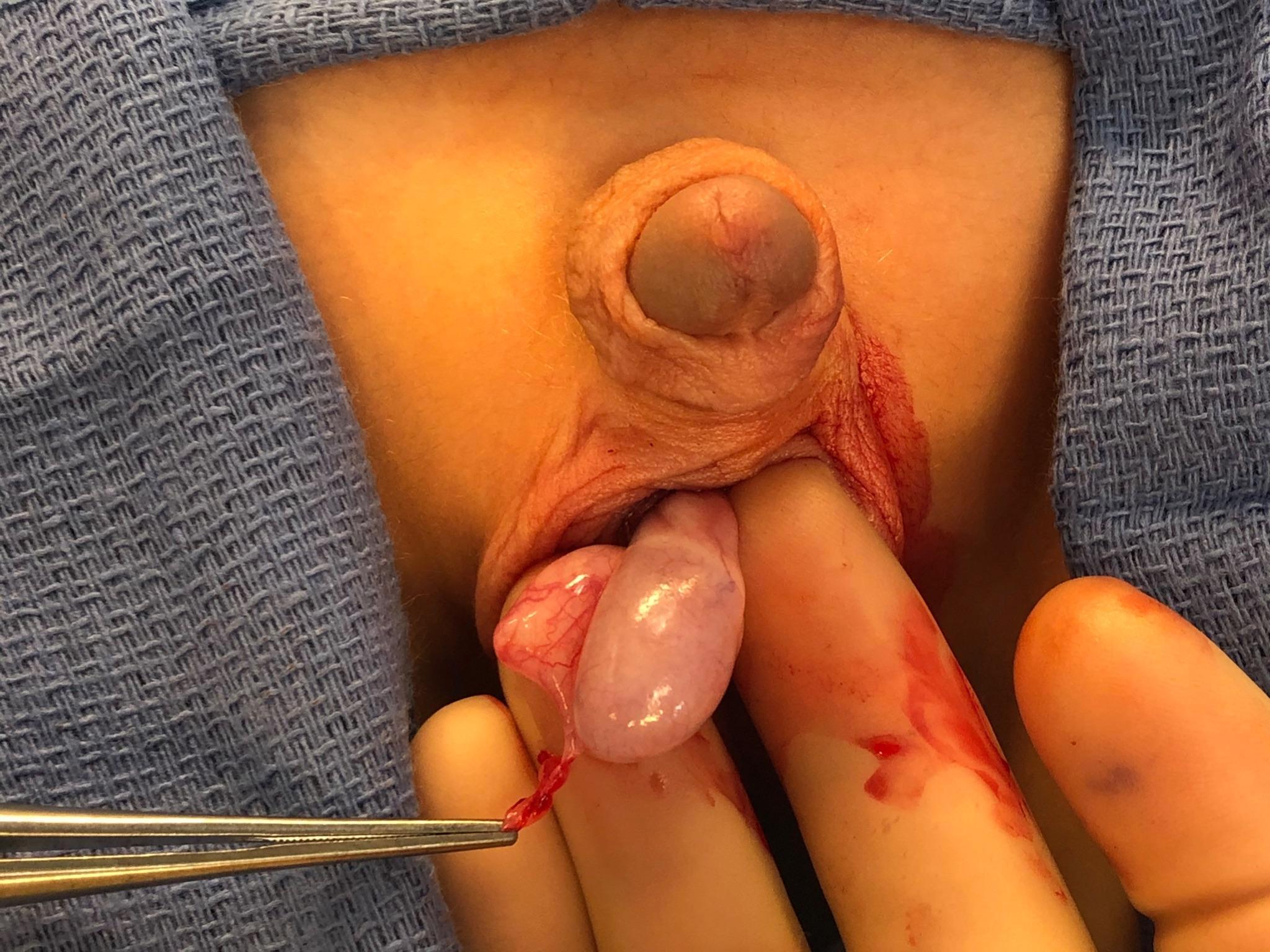
Figure 6 Forceps grasping a torsed appendix epididymis
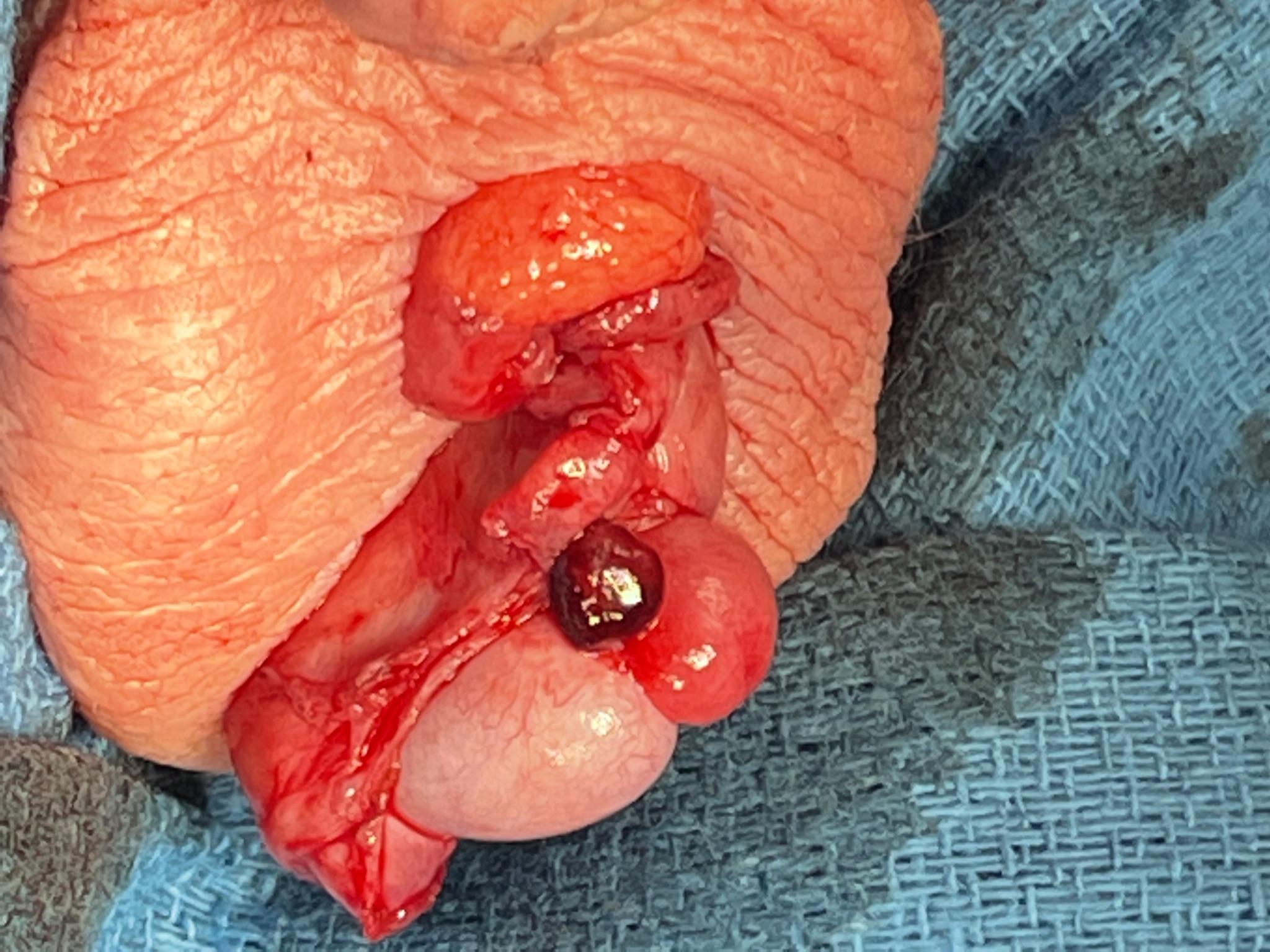
Figure 7 Appearance of an engorged appendix epididymis at scrotal exploration in what was thought to be an equivocal exam for TT
Epididymitis
Epidemiology
Epididymitis is inflammation of the epididymis: the coiled, tubular structure which sits posteriorly and superiorly upon the oval-shaped testis. The epididymis functions to transport and mature sperm from the testis. In adolescents, epididymitis accounts for 35-71% of cases of acute scrotal pain.37 While the condition occurs most frequently in sexually active older adolescents, it may also occur in younger patients secondary to viral infection or chemical (urine) inflammation.
Pathogenesis
In older patients, epididymitis most commonly occurs due to bacterial infection. Typically, a sexually transmitted infection (STI) is the culprit, especially in younger aged men, which results in migration of bacteria from the urethra into the vas and epididymis. Neisseria gonorrhea and Chlamydia trachomatis are the most common infectious agents in this population. Similarly, infected urine from a urinary tract infection may reflux into the vas deferens and cause epididymitis. This may be precipitated by heavy lifting.
In younger children, the most common cause of epididymitis is sterile and secondary to pressurized voiding of “sterile” urine to the ejaculatory ducts and back through the vas deferens to the epididymis (so called “sterile” or “chemical” epididymitis). Less common etiologies are secondary to viral infections (e.g., both named, like mumps orchitis secondary to Paramyxoviridae virus, or unnamed viruses), trauma, and tuberculosis.
Evaluation and Diagnosis
The diagnosis of epididymitis is clinical and often one of exclusion. A patient presenting with gradual onset of scrotal pain, scrotal swelling, and (possibly) fever should raise a high index of suspicion for epididymitis. They may also report symptoms of dysuria, urinary frequency, urgency, incontinence, and/or urethral discharge. In young children in whom chemical epididymitis is suspected, a careful history of voiding and constipation should be elicited. Parents will often report these children have long periods of urine holding followed by urinary urgency. Constipation may lead boys to both pressurized urine and stool voiding, both of which can pressurize urine reflux down the ejaculatory duct and vas deferens.
Physical exam should reveal a normal, vertical lie of the testis, normal cremasteric reflex, and relief of pain with elevation of the testis (Prehn sign). Palpation of the epididymis on the posterior aspect of the testis will elicit tenderness.
Work-up should include urinalysis and urine culture. In older patients with a concerning history or exam, CDUS may be necessary to rule out TT and other pathology, from testicular rupture to scrotal abscesses. In younger children in whom chemical epididymitis is expected, 1) bladder ultrasound assessing bladder distention and/or elevated post-void residual, and 2) plain film of the abdomen assessing for constipation can add support to the diagnosis.
Treatment
Treatment is variable and depends upon the cause of epididymitis. The primary treatment for epididymitis caused by STI is appropriate antimicrobials. Both ceftriaxone and doxycycline should be administered to treat N. gonorrhea and C. trachomatis. Azithromycin may be substituted for doxycycline in patients with an allergy or contraindication. Additional supportive care includes non-steroidal anti-inflammatory analgesics and scrotal support. Partners should be evaluated and treated with antibiotics, as well. Treatment for chemical epididymitis in a young child includes supportive care with scheduled non-steroidal anti-inflammatory analgesics and supportive underwear. Urinary holding, pressurized urine voiding, and constipation need to be addressed concomitantly.
Conclusion
In summary, an “acute scrotum” is the clinical descriptor of the rapid onset of a painful or swollen scrotum. The differential diagnosis includes but is not limited to testicular torsion, torsion of the appendix testes, and epididymitis. Testicular torsion should be ruled out prior to the workup of alternative etiologies.
Key Points
- The incidence of testicular torsion (TT) has a bimodal distribution: the first year of life (~1 month of age), when torsion is more likely to be extravaginal, and the pubertal period (~12 years of age) when torsion is more likely to be intravaginal
- The bell clapper deformity is the primary risk factor predisposing to intravaginal torsion
- TT is a clinical diagnosis: a focused history and physical exam are the only prerequisites for surgical exploration
- In cases with equivocal clinical findings, color doppler ultrasonography (CDUS) may be utilized to confirm end-organ ischemia
- The presence of vascular flow on CDUS does not necessarily rule-out the presence of developing ischemia
- Imaging should not be performed if it significantly delays surgical exploration and definitive treatment
- Be wary of isolated abdominal pain and remember to perform a genitourinary exam. Isolated abdominal pain as the sole complaint in a male child has been shown to be significantly associated with delayed presentations (>24 hours) and higher rates of orchiectomy
References
- Jefferies MT, Cox AC, Gupta A, Proctor A. The management of acute testicular pain in children and adolescents. Bmj 2015; 350 (apr02 5): h1563–h1563. DOI: 10.1136/bmj.h1563.
- Bayne CE, Villanueva J, Davis TD, Pohl HG, Rushton HG. Factors Associated with Delayed Presentation and Misdiagnosis of Testicular Torsion: A Case-Control Study. J Pediatr 2017; 186: 200–204. DOI: 10.1016/j.jpeds.2017.03.037.
- McCombe AW, Scobie WG. Torsion of Scrotal Contents in Children. J Urol 1988; 140 (1): 214–214. DOI: 10.1016/s0022-5347(17)41551-5.
- Kadish HA, Bolte RG. A Retrospective Review of Pediatric Patients With Epididymitis, Testicular Torsion, and Torsion of Testicular Appendages. Pediatrics 1998; 102 (1): 73–76. DOI: 10.1542/peds.102.1.73.
- Caesar RE, Kaplan GW. The Incidence of the Cremasteric Reflex in Normal Boys. J Urol 1994; 152 (2 Part 2): 779–780. DOI: 10.1016/s0022-5347(17)32707-6.
- Bingöl-Koloğlu M, Tanyel FC, Anlar B, Büyükpamukçu N. Cremasteric reflex and retraction of a testis. J Pediatr Surg 2001; 36 (6): 863–867. DOI: 10.1053/jpsu.2001.23956.
- Williamson RCN. Torsion of the testis and allied conditions. Br J Surg 1976; 63 (6): 465–476. DOI: 10.1002/bjs.1800630618.
- Zhao LC, Lautz TB, Meeks JJ, Maizels M. Pediatric Testicular Torsion Epidemiology Using a National Database: Incidence, Risk of Orchiectomy and Possible Measures Toward Improving the Quality of Care. J Urol 2011; 186 (5): 2009–2013. DOI: 10.1016/j.juro.2011.07.024.
- Mano R, Livne PM, Nevo A, Sivan B, Ben-Meir D. Testicular Torsion in the First Year of Life – Characteristics and Treatment Outcome. Urology 2013; 82 (5): 1132–1137. DOI: 10.1016/j.urology.2013.07.018.
- Mathews John C, Kooner G, Mathew DE, Ahmed S, Kenny SE. Neonatal testicular torsion – a lost cause? Acta Paediatr 2008; 97 (4): 502–504. DOI: 10.1111/j.1651-2227.2008.00701.x.
- Taghavi K, Dumble C, Hutson JM, Mushtaq I, Mirjalili SA. The bell-clapper deformity of the testis: The definitive pathological anatomy. J Pediatr Surg 2021; 56 (8): 1405–1410. DOI: 10.1016/j.jpedsurg.2020.06.023.
- Caesar RE, Kaplan GW. Incidence of the bell-clapper deformityin an autopsy series. Urology 1994; 44 (1): 114–116. DOI: 10.1016/s0090-4295(94)80020-0.
- Martin AD, Rushton HG. The Prevalence of Bell Clapper Anomaly in the Solitary Testis in Cases of Prior Perinatal Torsion. J Urol 2014; 191 (5s): 1573–1577. DOI: 10.1016/j.juro.2013.09.013.
- O’Kelly F, Chua M, Erlich T, Patterson K, DeCotiis K, Koyle MA. Delaying Urgent Exploration in Neonatal Testicular Torsion May Have Significant Consequences for the Contralateral Testis: A Critical Literature Review. Urology 2021; 153: 277–284. DOI: 10.1016/j.urology.2020.10.064.
- Erlich T, Ghazzaoui AE, Pokarowski M, O’Kelly F, Lorenzo AJ, Bagli DJ, et al.. Perinatal testicular torsion: The clear cut, the controversial, and the "quiet" scenarios. J Pediatr Surg 2021; 57 (10): 288–297. DOI: 10.1016/j.jpedsurg.2021.10.003.
- Bayne CE, Hsieh MH. Testicular Torsion. In: Cabana MD, editor. The 5-minute Pediatric Consult. 8th. DOI: 10.1016/b978-0-323-47778-9.50163-x.
- Srinivasan A, Cinman N, Feber KM, Gitlin J, Palmer LS. Faculty Opinions recommendation of History and physical examination findings predictive of testicular torsion: an attempt to promote clinical diagnosis by house staff. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2011; 7: 470–474. DOI: 10.3410/f.11135959.12106058.
- Boettcher M, Bergholz R, Krebs TF, Wenke K, Aronson DC. Clinical Predictors of Testicular Torsion in Children. Urology 2012; 79 (3): 670–674. DOI: 10.1016/j.urology.2011.10.041.
- Beni-Israel T, Goldman M, Bar Chaim S, Kozer E. Clinical predictors for testicular torsion as seen in the pediatric ED. Am J Emerg Med 2010; 28 (7): 786–789. DOI: 10.1016/j.ajem.2009.03.025.
- Barbosa JA, Tiseo BC, Barayan GA. Development and Initial Validation of a Scoring System to Diagnose Testicular Torsion in Children. Yearbook of Urology 2013; 2013: 6–7. DOI: 10.1016/j.yuro.2013.06.025.
- Sheth KR, Keays M, Grimsby GM, Granberg CF, Menon VS, DaJusta DG, et al.. Diagnosing Testicular Torsion before Urological Consultation and Imaging: Validation of the TWIST Score. J Urol 2016; 195 (6): 1870–1876. DOI: 10.1016/j.juro.2016.01.101.
- Jefferson RH, Perez LM, Joseph DB. Critical Analysis of the Clinical Presentation of Acute Scrotum: A 9-Year Experience at a Single Institution. J Urol 1997; 158 (3): 1198–1200. DOI: 10.1016/s0022-5347(01)64426-4.
- Friedman N, Pancer Z, Savic R, Tseng F, Lee MS, Mclean L, et al.. Accuracy of point-of-care ultrasound by pediatric emergency physicians for testicular torsion. J Pediatr Urol 2019; 15 (6): 608.e1–608.e6. DOI: 10.1016/j.jpurol.2019.07.003.
- Karmazyn B, Steinberg R, Kornreich L, Freud E, Grozovski S, Schwarz M, et al.. Clinical and sonographic criteria of acute scrotum in children: a retrospective study of 172 boys. Pediatr Radiol 2005; 35 (3): 302–310. DOI: 10.1007/s00247-004-1347-9.
- Bandarkar AN, Blask AR. Testicular torsion with preserved flow: key sonographic features and value-added approach to diagnosis. Pediatr Radiol 2018; 48 (5): 735–744. DOI: 10.1007/s00247-018-4093-0.
- Kalfa N, Veyrac C, Lopez M, Lopez C, Maurel A, Kaselas C, et al.. Multicenter Assessment of Ultrasound of the Spermatic Cord in Children With Acute Scrotum. J Urol 2007; 177 (1): 297–301. DOI: 10.1016/j.juro.2006.08.128.
- NUSSBAUM BLASK ANNAR, BULAS DOROTHY, SHALABY-RANA EGLAL, RUSHTON GIL, SHAO CHENG, MAJD MASSOUD. Color Doppler sonography and scintigraphy of the testis: A prospective, comparative analysis in children with acute scrotal pain. Pediatr Emerg Care 2002; 18 (2): 67–71. DOI: 10.1097/00006565-200204000-00001.
- Paltiel HJ, Connolly LP, Atala A, Paltiel AD, Zurakowski D, Treves ST. Acute Scrotal Symptoms in Boys With an Indeterminate Clinical Presentation: Comparison of Color Doppler Sonography and Scintigraphy. J Urol 1998; 161 (4): 1408–1408. DOI: 10.1016/s0022-5347(01)61728-2.
- SESSIONS ANNETTEE, RABINOWITZ RONALD, HULBERT WILLIAMC, GOLDSTEIN MARTINM, MEVORACH ROBERTA. Testicular Torsion: Direction, Degree, Duration and Disinformation. J Urol 2003; 169: 663–665. DOI: 10.1097/00005392-200302000-00059.
- Castañeda-Sánchez I, Tully B, Shipman M, Hoeft A, Hamby T, Palmer BW. Testicular torsion: A retrospective investigation of predictors of surgical outcomes and of remaining controversies. J Pediatr Urol 2017; 13 (5): 516.e1–516.e4. DOI: 10.1016/j.jpurol.2017.03.030.
- Mellick LB, Sinex JE, Gibson RW, Mears K. A Systematic Review of Testicle Survival Time After a Torsion Event. Pediatr Emerg Care 2019; Publish Ahead of Print: 821–825. DOI: 10.1097/pec.0000000000001287.
- Dias Filho AC, Oliveira Rodrigues R, Riccetto CLZ, Oliveira PG. Improving Organ Salvage in Testicular Torsion: Comparative Study of Patients Undergoing vs Not Undergoing Preoperative Manual Detorsion. J Urol 2017; 197 (3 Part 1): 811–817. DOI: 10.1016/j.juro.2016.09.087.
- Garel L, Dubois J, Azzie G, Filiatrault D, Grignon A, Yazbeck S. Preoperative manual detorsion of the spermatic cord with Doppler ultrasound monitoring in patients with intravaginal acute testicular torsion. Pediatr Radiol 2000; 30 (1): 41–44. DOI: 10.1007/s002470050012.
- Yecies T, Bandari J, Schneck F, Cannon G. Direction of Rotation in Testicular Torsion and Identification of Predictors of Testicular Salvage. Urology 2018; 114: 163–166. DOI: 10.1016/j.urology.2017.11.034.
- Kutikov A, Casale P, White MA, Meyer WA, Chang A, Gosalbez R, et al.. Testicular Compartment Syndrome: A New Approach to Conceptualizing and Managing Testicular Torsion. Urology 2008; 72 (4): 786–789. DOI: 10.1016/j.urology.2008.03.031.
- Lev M, Ramon J, Mor Y, Jacobson JM, Soudack M. Sonographic appearances of torsion of the appendix testis and appendix epididymis in children. J Clin Ultrasound 2015; 43 (8): 485–489. DOI: 10.1002/jcu.22265.
- Lehmann C, Biro FM, Slap GB. Chapter 20 - Testicular and Scrotal Disorders. Adolescent Medicine. Philadelphia: Mosby; 2008.
Última actualización: 2023-02-22 17:22