29: Congenital Hydrocele and Inguinal Hernia
This chapter will take approximately 11 minutes to read.
Introduction
The following chapter will try to describe in a complete, yet concise and summarized way about the history, embryology and development, diagnosis and treatment of congenital inguinal hernia and hydrocele. We will emphasize details related to different aspects of the topic, offering useful tips for the reader for the appropriate approach to the pathology.
History
The timeline described below emphasizes historical milestones and advances overtime related to congenital inguinal hernia and hydrocele (Figure 1)
In the year 25 AC, Celsus gave the first reference to hernia repair in children.
Advances in medicine as well as post operative patient care have improved surgical techniques and surgical outcomes over time. Nowadays the surgical procedure is done similarly worldwide with minimal variations depending on the surgeon preferences and the chosen approach (laparoscopic vs conventional) for repair.
Figure 1 Timeline
Embryology
Hydrocele and congenital inguinal hernia appear as a result of failure in the process of obliteration of the processus vaginalis (PV), a peritoneal evagination created from coelomic epithelium which normally seals during the first year of age.
Hydrocele could be defined as a collection of fluid between the parietal and visceral layers of the tunica vaginalis (TV) formed when the PV remains patent. They may be classified as communicating, non-communicating, and cord hydrocele. If the lumen is wide enough, abdominal contents may be extruded into it and create an inguinal hernia.1
At the final stage of fetal life, the testes and ovaries will occupy a different position from what they did have in the embryo. The change in position of the testes is much more pronounced than that of the ovaries and includes its journey through the thickness of the abdominal wall to the scrotum.2
The events that govern testicular development and subsequent descent in males are:
3rd Week
The primordial germ cells (extra-embryonic gametes), which are in the wall of the yolk sac, migrate towards the gonadal ridges (Figure 2)
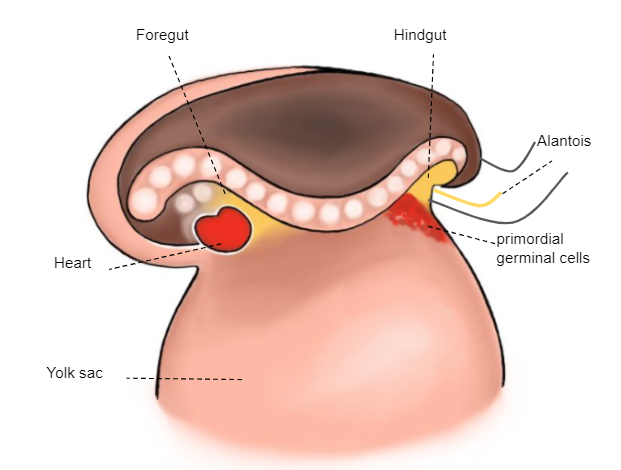
Figure 2 3rd week embryo
6th Week
The undifferentiated gonad is attached to the posterior wall by the mesoperitoneum that, cranially, forms the diaphragmatic ligament and, caudally, the inguinal ligament (gubernaculum) (Figure 3)
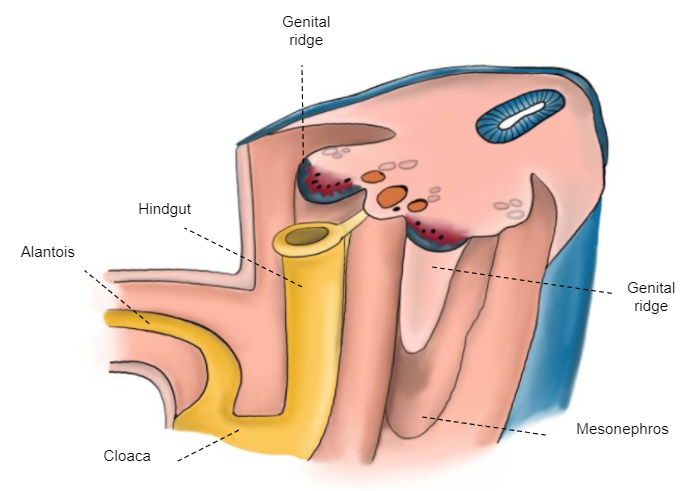
Figure 3 6th week embryo
7th–8th Week
The sex cords penetrate the medulla originating the testicular cords, which reach the mesorchium and form the rete testis (Haller's network). Later, these are transformed into efferent cords. (Figure 4)
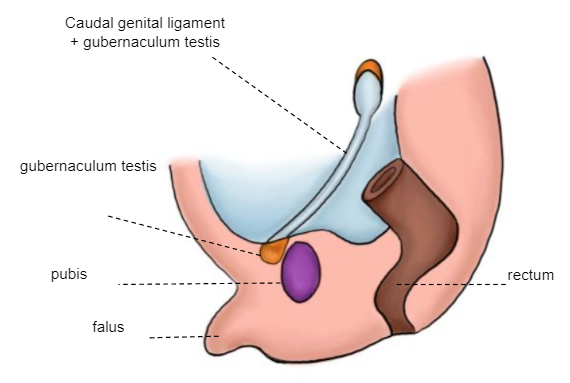
Figure 4 7–8th week embryo
10th–12th Week
The gubernaculum testis is attached distally to the labioscrotal ridge, which will serve as an axis around which the mesoderm is modeled, giving rise to the inguinal canal. A peritoneal evagination, processus vaginalis (coelomic epithelium), is formed following the gubernaculum’s trajectory towards the labioscrotal ridge. The first phase of the testicular descent occurs, in which the testis passes from its lumbar position to the vicinity of the external inguinal orifice (Figure 5)
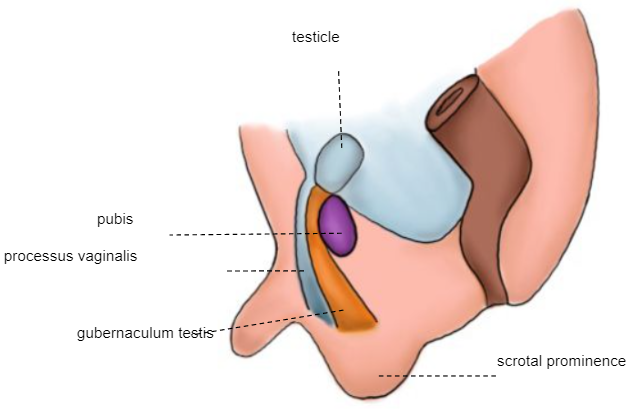
Figure 5 10–12th week embryo
28th Week
Second phase of descent: the testicle passes through the already formed inguinal canal, reaching the scrotum between weeks 32 and 35 (Figure 6)
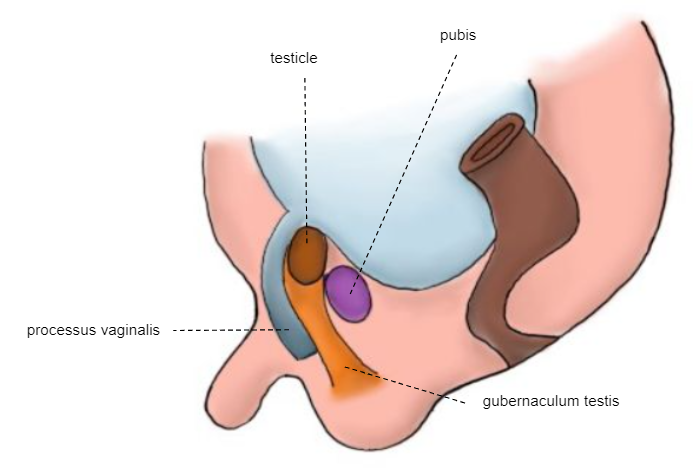
Figure 6 28th week embryo
Term Newborn
The testicle is fixed at the scrotum (Figure 7)
The mechanical factors that could trigger the testicular descent to the scrotum are:
- Distal traction generated by the gubernaculum testis.
- Intra-abdominal pressure.
Other possible determining factors include hormonal and neurological factors.2
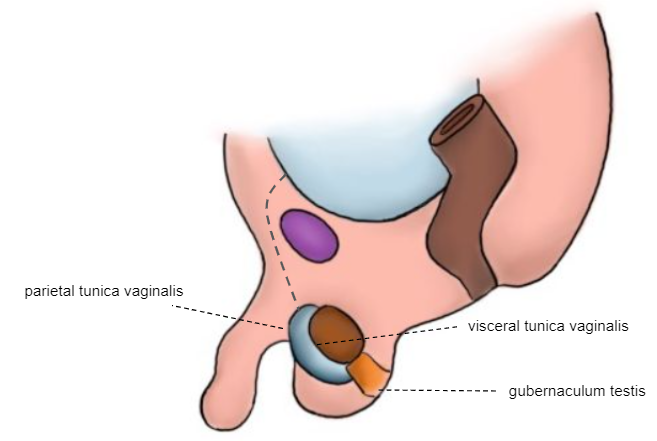
Figure 7 Newborn
As shown in the previous successive embryological illustrations, the processus vaginalis (PV) begins to obliterate shortly before birth. Complete closure is normally achieved during the first year of life. The tunica vaginalis (TV) remains surrounding the testis. Failure in the process of obliteration of the PV is then associated with hernia, hydrocele, and encysted cord hydrocele.
Epidemiology
The incidence of congenital indirect inguinal hernia in full-term neonates is 3.5–5%, while in preterm infants it is considerably higher and ranges from 9–11%. It approaches 60% as birth weight decreases from 500 to 750 grams. Most series report a male preponderance over females ranging from 5:1 to 10:1.3
On the other hand, communicating hydroceles are common in newborns with an incidence of 2–5%; 90% of these resolve during the first year of life as a result of spontaneous closure.4
Congenital inguinal hernias are more common on the right side (60%), 30% occur on the left side and 10% are bilateral. This distribution does not change with sex as even in girls a right-side-predominance exists.5
Etiology / Pathogenesis
The anatomy of the inguinal canal varies slightly with age. In adults and children, the internal and external inguinal orifices are widely separated, whereas in young infants they practically overlap. In girls, the anatomy is similar except for the absence of spermatic elements which are replaced by the round ligament.3 The different types of hydroceles are illustrated in the image below (Figure 8)
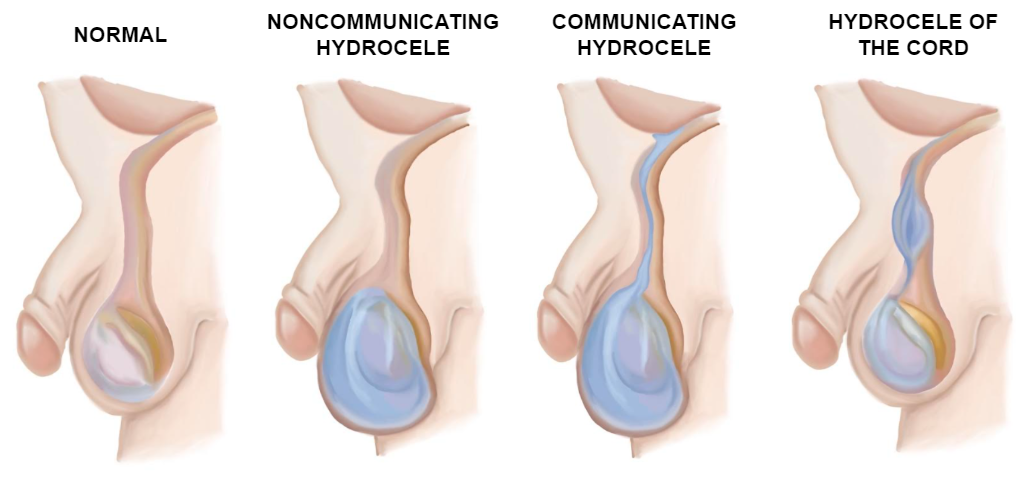
Figure 8 Types of hydroceles
Congenital indirect inguinal hernia can be formed, as it was already mentioned, from a permeable PV allowing the extrusion of omentum, intestines, appendix or even ovaries or fallopian tube in females through the inguinal canal. A hernial sac may as well extend from the abdominal cavity, through the internal inguinal ring down to the TV, resulting in the so-called inguinoscrotal hernia.
Direct inguinal hernias are very uncommon in the pediatric population, they are mostly seen in adults caused by posterior abdominal muscle weakness enabling intestines to slide into the groin.
Diagnosis and Evaluation
Inguinal hernia and hydrocele can be diagnosed by physical exam without the strict use of images. The presence of a reducible asymptomatic firm bulge in the groin or scrotum in males or a mass in the labia majora in girls is suspicious of an inguinal hernia.
Incarceration of the hernial sac should be suspected in a patient who presents with severe, sudden onset of pain and a hard, tender, fixed mass in the groin. If it is not reduced manually surgical reduction should follow.6,7
Physical exam should include evaluation of the patient in a supine position, reinforcing, if possible, the Valsalva maneuver to identify transient hernias; and the standing-up evaluation where the physician should palpate and compare both groins looking for thickening of the inguinal cord or a bulge. In males, correct descent of testicles should be checked and transillumination (light source over the scrotum showing absence of the testicular shadow) can sometimes help to confirm hydrocele.
Tips
- During palpation, it is most important to feel the inguinal cord slide under the finger pads.
- We suggest palpating the inguinal cord with the most skilled hand.
- In neonates, hernias can be more often reduced. This should be done with compression of the herniated content upwards using the skilled hand while exerting pressure downwards with the other hand as to align both inguinal rings and direct herniated elements into the abdominal cavity (Figure 9)
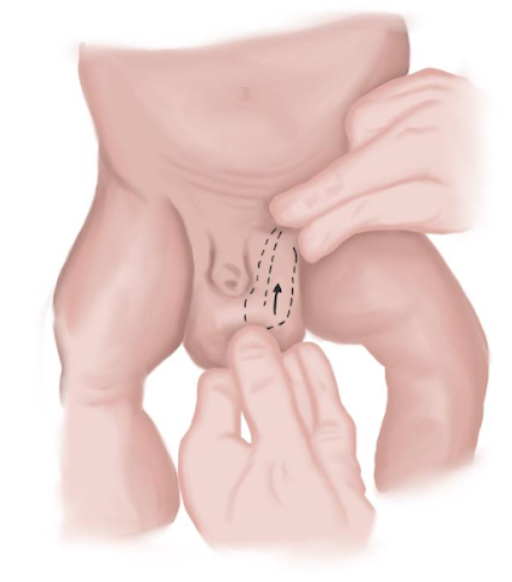
Figure 9 Hernia reduction maneuver in neonates.
If the physical exam is not confirmative, inguinal ultrasound may add information to the suspected diagnosis.8 Nonetheless, the physical exam is still the gold standard diagnostic method. The sensibility of ultrasound depends mainly on the operator’s skills and cooperation from the patient (stillness and Valsalva maneuver). The hernia contents can be hyperechoic due to omental fat, anechoic in the presence of fluid, or of mixed echogenicity with reverberations which correlates with the existence of air in the bowel loops’ lumen.9 The US could also rule out a contralateral inguinal hernia, or PV that could be repair in the same surgery.
Differential Diagnosis
Differential diagnosis for inguinal lesions is often challenging due to semiological similarities to the physical exam. Once a tender palpable mass is detected, the physician should have in mind the following possible diagnosis making use of other complementary studies if a physical exam is not enough:
- Undescended testis.
- Hematoma / Inflammation.
- Abscess.
- Benign or malignant tumors / Metastatic or benign lymph node enlargement.
- Round ligament varicosities.
- Mesothelial cyst.
Treatment Options
The treatment of inguinal hernia is always surgical, and the key aspect of treatment is to achieve a high ligation of the hernial sac.
In infants and toddlers, herniotomy can be performed through the external inguinal orifice without opening the aponeurosis as both orifices usually overlap. In older patients’ orifices are separate from each other. In such cases, it is advisable to open the external oblique aponeurosis in order to achieve a high ligation of the sac.3 The use of parietal mesh in pediatric patients is reserved for special cases.
For both hydrocele and inguinal hernias, surgical principles and techniques are very similar. When hydrocele is surgically treated, in addition to the high ligation of the sac, the distal aspect of it should be resected as well. This step is not imperative for inguinal hernias.
Inguinal hernias can be approached by either an open inguinal herniorrhaphy (OIH) or a laparoscopic inguinal hernia repair (LIHR). The most important components of the procedure involve ensuring the hernial sac is emptied and entirely closed after surgery, preserving the integrity of vas deferens, testicular vessels, and ilioinguinal nerve. In girls, round ligament fixation should be done to ensure correct uterine fixation preventing future dyspareunia.
Open Inguinal Herniorrhaphy
The main steps of OIH are detailed below with schematic drawings of the maneuvers.
- Transverse inguinal incision flowing the natural skin fold over the cord (Figure 10)
- Dissection of the subcutaneous cellular tissue incising the scarpa fascia until the muscular aponeurosis is reached.
- Incision over the muscular aponeurosis (not always needed) (Figure 11)
- Extension of aponeurosis incision toward the superficial inguinal orifice.
- The inguinal cord is then dissected on both sides and on the underside with blunt maneuvers. The cord is then repaired with an elastic loop.z
- Cremasteric muscle is teased open by blunt dissection on the anteromedial surface of the cord and spread to expose the glistening peritoneum of the indirect hernia sac (Figure 12)
- The cord and its contents (vessels, vas deferens) are dissected gently, separating it from the sac that will be in the anteromedial situation.
- The cord´s contents are carefully set apart from the sac which is detached from the latter (Figure 13)
- The hernial sac is opened and its content reduced to the abdominal cavity.
- The hernial sac is twisted ensuring there is no content inside of it. An absorbable polyglactin knot is tied on its base and the sac is cut and lost into the abdominal cavity (Figure 14), (Figure 15), and (Figure 16)
- Muscular Aponeurosis is closed with a continuous absorbable polyglactin suture if it is opened (Figure 17)
- Separated knots are used to approximate subcutaneous tissue and an intradermal suture is used to approximate skin tissue (Figure 18)
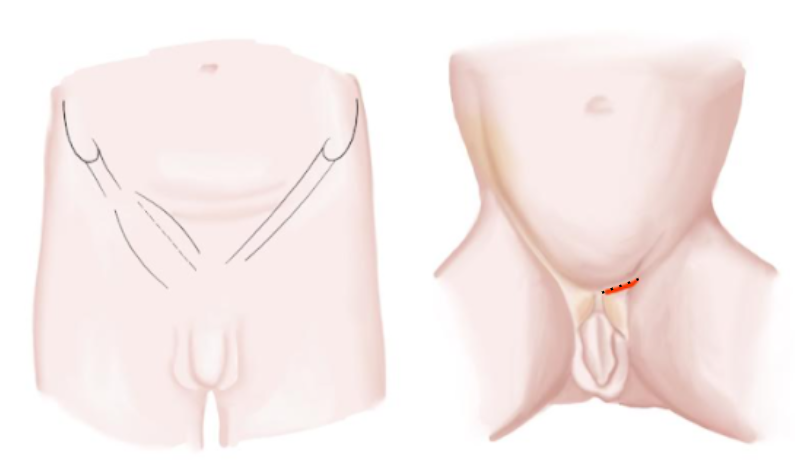
Figure 10 Inguinal incision infants and adolescents
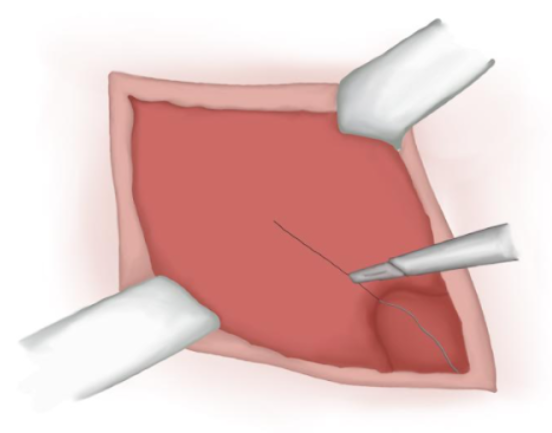
Figure 11 Incision over the muscular aponeurosis.
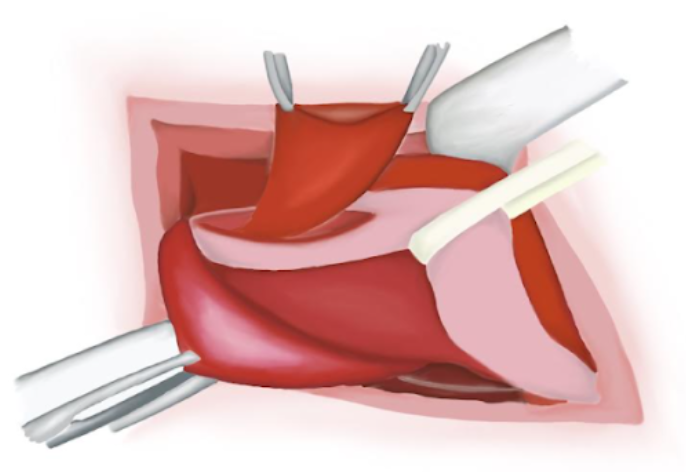
Figure 12 Inguinal cord identification. Hernial sac dissection.
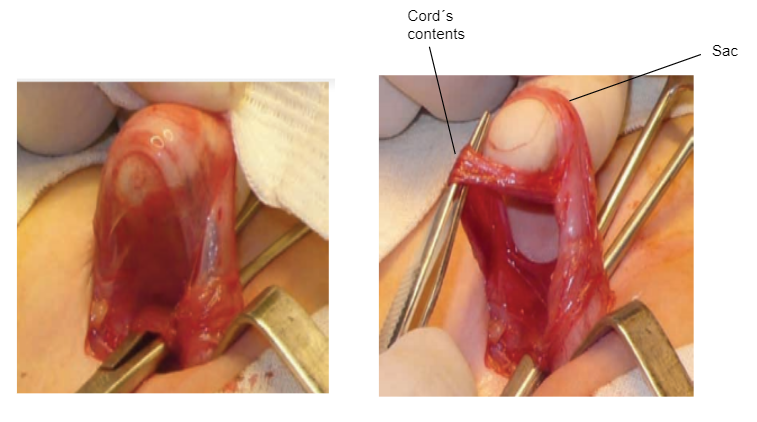
Figure 13 Hernial sac dissection.
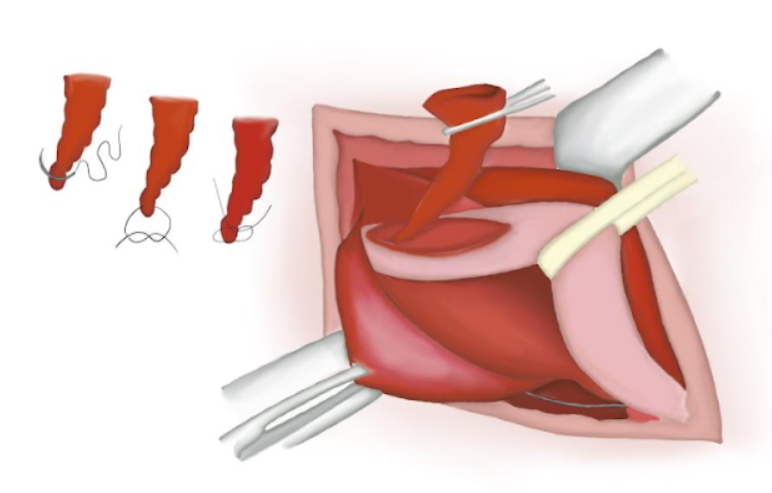
Figure 14 Hernial sac is twisted ensuring no content is present inside.
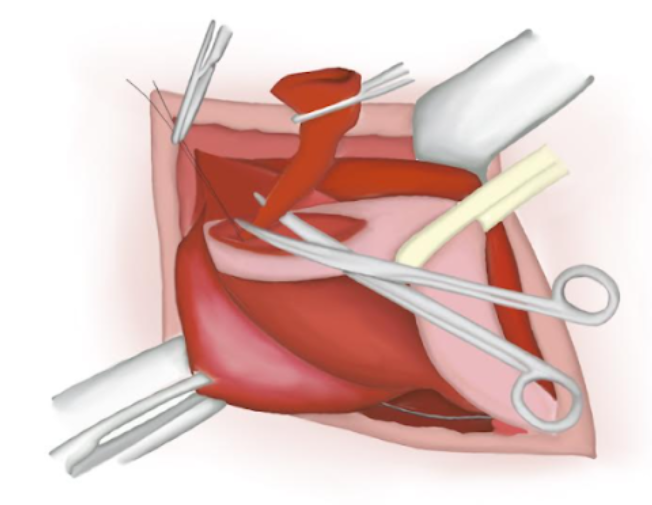
Figure 15 Hernial sac resected.
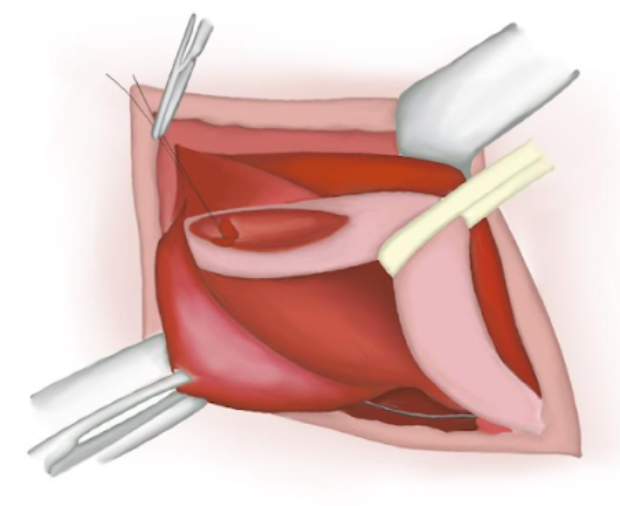
Figure 16 Sutured proximal end lost into the abdominal cavity.
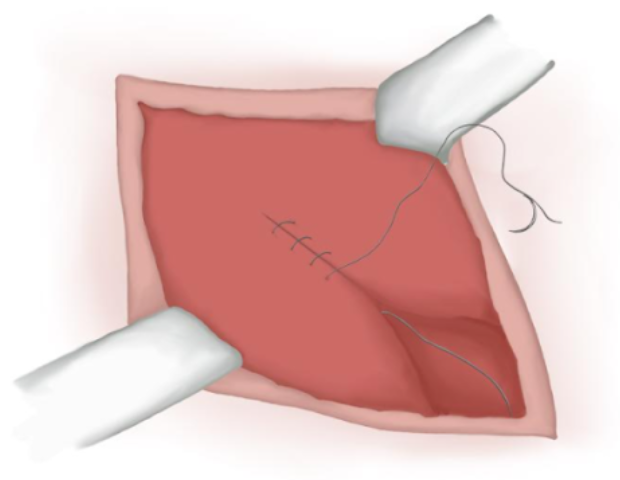
Figure 17 Muscular aponeurosis closure.
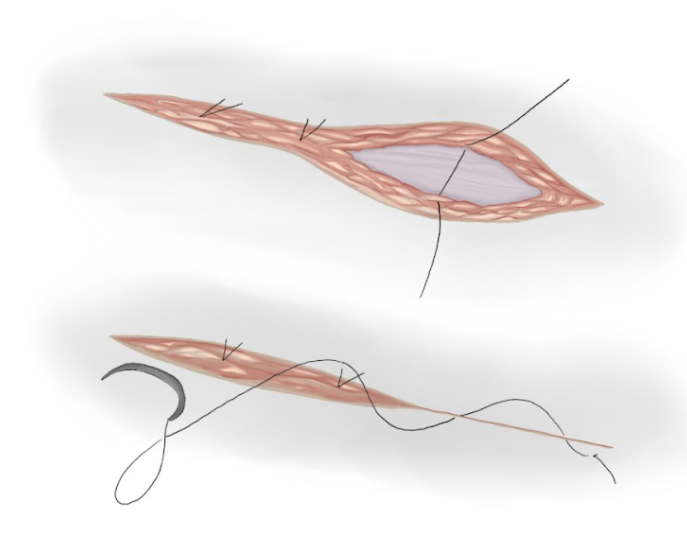
Figure 18 Subcutaneous and skin closure.
Tips
- The authors recommend a transverse skin incision following skin fold.
- Locate the femoral arch in the aponeurotic plane in more complex cases (girls with a bilateral hernia, obese children) to locate the superficial inguinal orifice.
- Dissect the hernial sac always on the same side of the cord in a regulated manner
Laparoscopic Inguinal Hernia Repair
The role of laparoscopic repair of inguinal hernias is not fully established. The approach is criticized for transforming an extraperitoneal procedure into a transperitoneal one. Detractors sustain that this technique implies a technically more complex surgery and that it results in a higher recurrence rate than the conventional approach.
In contrast, the advocates of the laparoscopic approach stress on the fact that this technique ensures reduced invasiveness, without manipulation of the chordal structures, and it can be useful for the correct diagnosis of associated direct and femoral hernias. Moreover, the contralateral internal ring can be clearly visualized and repaired if necessary. Finally, supporters endorse the improved cosmesis resulting after this surgery compared with a conventional incision (Table 1).10
Table 1 The following table exhibits some advantages and disadvantages regarding LIHR:
| ADVANTAGES | DISADVANTAGES |
|---|---|
| Easy-going technique. | Costs. |
| Outpatient procedure. | Could be a longer surgery duration. |
| Cord structures remain untouched. | General anesthesia with endotracheal intubation is necessary. |
| Type of hernia is evident as well as the contralateral inguinal ring. | Increased possibility of injury to intra abdominal organs. |
| Clear visualization of the anatomy. | Longer learning curve. |
| Inspection of the adnexal in girls. | Possible cord damage in the long term |
Post-Operative Care and Follow-Up
As this is a one-day case surgery, postoperatively food and drinks can be initiated after recovery from the anesthetic. Infants with bronchopulmonary dysplasia, anemia, prematurity, or those who required ventilatory support at the time of birth, should be observed after operative repair for at least 24 hours, monitored for episodes of apnea and/or bradycardia. Patients should be discharged with oral analgesics.
Activity should be limited for 48 hours. Returning to normal activities as soon as the child feels capable. In children older than 6 years, activity restrictions should be more strict with no vigorous activity for 1 month period. Baths can be initiated the third day after surgery.
Follow-up should be done 1 week after surgery for early complications followed by by clinical controls 1 month, 3 and 6 months post surgery before discharge.
Outcomes, Complications, and Management
During surgery, local complications include bleeding and injury to spermatic vessels and vas deferens. The use of magnifying loupes during the reconstruction of intraoperative injuries will make the repair more precise, especially in newborns.
Early complications after surgery may include local edema, hematoma, and wound infection.
Late complications seen during follow-up may include recurrent hydrocele or hernia, iatrogenic displacement of the testis, and testicular atrophy. To avoid postoperative hydrocele, the anterior and lateral aspects of the sac can be partially resected, or the distal sac can be open completely.
Recurrence is higher in males that are operated before the first year of age and those managed by laparoscopic approach. Recurrence reduces significantly when the procedure is done by a skilled experienced pediatric surgeon or pediatric urologist, or if it is a scheduled surgery.
Recurrent inguinal hernia in children may include:
- A missed hernial sac or unrecognized peritoneal tear.
- Foul ligation of the sac.
- Failure to repair a large internal inguinal ring.
- Unrecognized defect of the floor of the inguinal canal (direct inguinal hernia).
- Infection.
- Increased intra-abdominal pressure (patients with ventricular-peritoneal shunts or continuous ambulatory peritoneal dialysis).
- Patients with cystic fibrosis and chronic cough.
- Connective tissue disorders (i.e., Ehlers-Danlos syndrome).
Key Points
- Literature for inguinal hernia has spanned more than 20 centuries. Galen in 176 A.D. firstly described the pathogenesis of indirect inguinal hernia as he depicted for the first time the “Processus Vaginalis Peritonei”.
- The incidence of congenital indirect inguinal hernia in full-term neonates is 3.5–5%, while in preterm infants 9–11%.
- Epidemiology: Mostly seen in males, with a male to female ratio ranging from 5:1 to 10:1.
- Diagnosis of both inguinal hernia and hydrocele can be done by physical exam and confirmed without the strict use of images.
- Treatment of inguinal hernia is always surgical. They can be approached by either an open inguinal herniorrhaphy (OIH) or a laparoscopic inguinal hernia repair (LIHR).
- Surgical treatment is usually ambulatory. Infants with bronchopulmonary dysplasia, anemia, prematurity, or those who required ventilator support at the time of birth, should be observed after operative repair for at least 24 hours.
- Follow-up should be done 1 week after surgery for early complications followed by clinical controls 1 month, 3 and 6 months post surgery.
- Early complications after surgery may include local edema, hematoma, and wound infection.
- Late complications seen post-surgery during follow-up include recurrent hydrocele or hernia, iatrogenic displacement of the testis, and testicular atrophy.
Conclusions
Patent Processus Vaginalis (PV) leads to congenital hydrocele and inguinal hernia. Palpable mass in the groin should be evaluated for possible PV-associated conditions. Physical examination is the gold standard diagnostic method for these conditions. Treatment of inguinal hernia and communicating hydrocele with an associated hernia is always surgical. The success of surgical treatment depends on a high ligation of the sac as well as distal resection of it in hydroceles. Approaches include open inguinal herniorrhaphy as well as laparoscopic.
Complications may be seen during surgery or at an early or late postoperative stage, which includes edema, hematoma or recurrent hydrocele, or hernia respectively. Several strategies are described by experts to avoid them. Follow-up should be done periodically for at least 6 months.
Ongoing controversies on the management of hernia and hydrocele still exist, especially when it comes to surgical technique based on recurrence and complication rates, aiming for minimally invasive procedures with better outcomes.
References
- Gearhart JP, Rink RC, Mouriqand PDE, editors. Pediatric Urology. 2nd ed., Philadelphia, PA: Elsevier; 2010, DOI: 10.1097/00042307-200011000-00007.
- Palermo M. Hernias de la pared abdominal - Conceptos clásicos, evidencias y nuevas técnicas. Actualidades Médicas, C.A: AMOLCA; 2012.
- J. T. Chapter 59: Inguinal Hernia. In: Puri P, editor. Newborn Surgery. 2nd ed. Boca Raton, FL: CRC Press; 2003. DOI: 10.5005/jp/books/12263_1.
- Godbole P.P. MNP. Chapter 18: Testis, Hydrocele and Varicocele. In: Wilcox DT, Thomas DFM, editors. Essentials of Pediatric Urology. 2nd ed. Boca Raton, FL: CRC Press; 2021. DOI: 10.1201/9781003182023.
- Rowe MI, Clatworthy HW. The other side of the pediatric inguinal hernia. Surg Clin North Am 1971; 51: 1371. DOI: 10.1016/s0039-6109(16)39592-5.
- Choi KH, Baek HJ. Incarcerated ovarian herniation of the canal of Nuck in a female infant: Ultrasonographic findings and review of literature. Annals of Medicine and Surgery 2016; 9: 38–40. DOI: 10.1016/j.amsu.2016.06.003.
- Stringer MD, Oldham KT, P.D.E. M, editors. Pediatric Surgery and Urology Long-term Outcomes. 2nd ed., Cambridge, UK: Cambridge University Press; 2010.
- Park HR, Park SB, Lee ES, Park HJ. Sonographic evaluation of inguinal lesions. Clinical Imaging 2016; 40 (5): 949–955. DOI: 10.1016/j.clinimag.2016.04.017.
- Yang DM, Kim HC, Lim JW, Jin W, Ryu CW, Kim GY. Sonographic findings of groin masses. J Ultrasound Med 2007; 26: 605–614. DOI: 10.7863/jum.2007.26.5.605.
- Holcomb GW, Georgeson KE, Rothenberg SE, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Amsterdam, Netherlands: Elsevier; 2021, DOI: 10.1007/978-3-030-84467-7_61.
Last updated: 2024-02-16 21:59