19: 神经源性膀胱
阅读本章大约需要 7 分钟。
引言
先天性脊柱裂占小儿神经源性膀胱和肠道病例的绝大多数。1 涉及脊柱的神经源性功能障碍的其他病因包括骶骨缺如、脊髓栓系、泄殖腔畸形以及脊髓损伤。伴有中枢神经系统异常(如痉挛性双瘫[脑性瘫痪])的儿童也可能出现神经源性膀胱。
膀胱动力学异常可导致慢性肾脏病及一系列潜在后遗症。2 神经源性膀胱的管理包括在保护上尿路功能的同时,实现低压储尿并获得控尿。最佳治疗需要多学科医疗团队,以预防和处理可能影响功能、生活质量和生存的潜在后遗症。鉴于脊柱裂占神经源性膀胱的大多数病例,因此本章将以其为重点。
胚胎学
神经管缺陷(NTDs)是指在胎内发育第3和第4周神经管未能闭合所致的脑和脊髓畸形。3 当通过椎管壁缺损,脊髓连同脑膜暴露并/或向体表突出时,即发生脊柱裂。椎管的发育自妊娠第18天开始,于第35天完成,从身体头侧端开始向尾侧方向闭合。脊柱裂性畸形归因于头侧中胚层发育异常。3 中胚层未能在发育中的脊髓表面长入会导致开放性病变,最常见于腰骶部(表 1)。暴露的脊髓及其神经根(可突入脑膜膨出囊),以及随着胎儿延长时脊髓沿椎管向头侧上移所产生的牵张(妊娠中晚期位于L2、L3,至出生时为L1),共同导致下尿路和下肢的神经损伤表现多变。4 脑积水和阿诺德-基亚里II型畸形(脊髓脊膜膨出与小脑扁桃体疝的组合)常与脊柱裂相关。5
表 1 脊髓脊膜膨出的脊柱节段。
| 部位 | 发生率 |
|---|---|
| 颈段-上胸段 | 2% |
| 下胸段 | 5% |
| 腰段 | 26% |
| 腰骶段 | 47% |
| 骶段 | 20% |
流行病学
脊柱裂是最常见的神经管缺陷,在美国约每3,000例活产中有1例,在全球约每1,000例活产中有1例。6,7 尽管在美国对强化谷物制品实施叶酸强制性强化后,脊柱裂的发病率显著下降,但脊柱裂的人群负担仍在持续,既体现在出生患病率上,也体现在不均等的长期结局上。8,9,10 母亲的种族/族裔差异已被证实会影响脊柱裂的患病率,其中西班牙裔母亲的患病率高于白人和黑人女性。11 性别偏倚因国家而异;在美国,普遍认为脊柱裂在女孩中的患病率高于男孩。12
发病机制
脊柱裂通常在出生时即可见,表现为病变部位的裸露神经组织,伴或不伴突出的囊袋。脊髓脊膜膨出是指由于原始神经管闭合不全,脊髓自椎管突出进入充满液体的囊袋(图1) 神经功能缺损的范围和严重程度取决于沿神经轴线的病变位置 - 依据累及的节段不同,缺损处的脊髓中断可导致下肢瘫痪、大小便失禁、皮肤感觉丧失,以及髋、膝和足的畸形。13,14 对逼尿肌和括约肌的躯体、副交感与交感神经支配受累程度不一,从而影响膀胱的储尿与排尿能力 - 脊髓脊膜膨出几乎总是伴有神经源性膀胱。异常的膀胱动力学进而可导致慢性肾脏病及多种潜在的并发症与后遗症。2
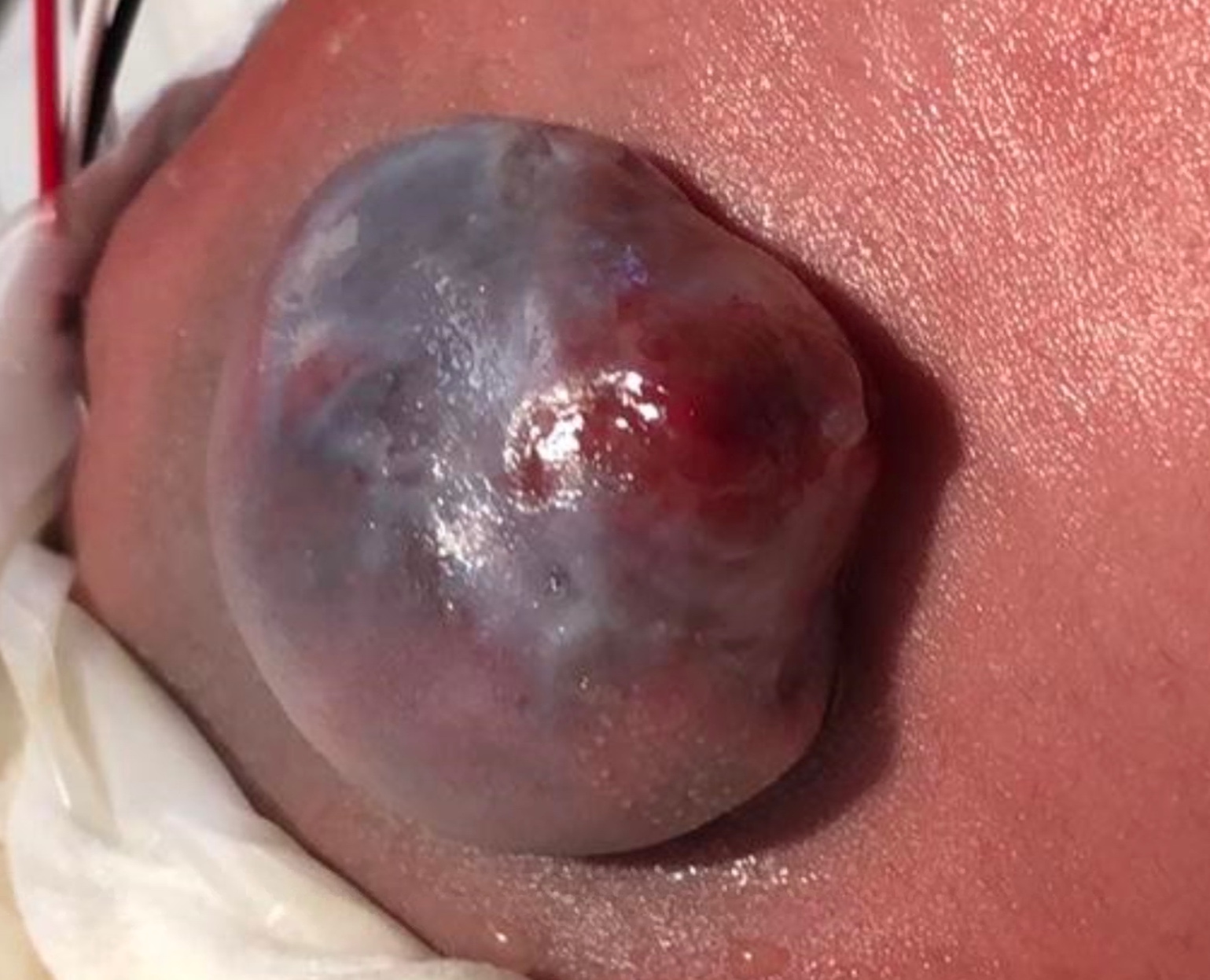
图 1 开放性脊髓脊膜膨出。
脊髓脊膜膨出通常与Chiari II型后脑畸形、脑室扩大和脑积水相关。15 Chiari II型畸形是指小脑蚓部向下移位进入颈椎椎管。5 其导致脑干延长和第四脑室闭塞,进而使脑脊液循环受阻,并在90%的患者中发生脑积水。5,13 约82%的病例需要治疗脑积水,治疗包括通过分流术将脑脊液引流至腹膜腔或其他体腔。16
评估与诊断
尽管目前尚不清楚如何为脊柱裂相关的膀胱功能障碍提供最佳护理,但近年来的趋势是对患有神经源性膀胱的儿童采取主动而非被动的管理。17 观察包括通过无创检查进行定期监测,包括肾-膀胱超声,以评估上尿路是否存在扩张/恶化。然而,许多小儿泌尿外科医师现在主张在婴儿期对下尿路进行全面评估,并在出生时即开始预防性治疗,或在初次尿动力学检查提示存在出口梗阻征象和/或膀胱充盈或排尿压力升高时启动预防性治疗。18,19,20
2012年,美国疾病控制与预防中心(CDC)召集了由小儿泌尿科医师、肾脏科医师、临床流行病学家、方法学专家、社区倡导者及CDC工作人员组成的工作组,制定标准化方案,以优化脊柱裂患儿自新生儿期至5岁期间的泌尿系统照护 (图2)21 行肾膀胱超声以确定上尿路扩张的基线情况。17 在3个月龄前完成视频尿动力学检查 – 透视图像可显示是否存在膀胱输尿管返流,以及膀胱轮廓不规则/小梁形成 (图3)]。出生时的首要问题是是否存在逼尿肌-括约肌协同障碍,以及婴儿能否在低压力下排空。逼尿肌充盈压力升高、膀胱括约肌协同障碍或漏点压力升高(>40 cm H20)可导致上尿路损害,需及早采用清洁间歇性导尿(CIC)和抗胆碱药进行干预。22,23,24,25 在积极管理下,婴儿按计划每6小时进行一次清洁间歇性导尿(CIC) – 若导尿量持续≤30 mL,可降低导尿频率;关键在于家属在出院前学会实施导尿。21,26
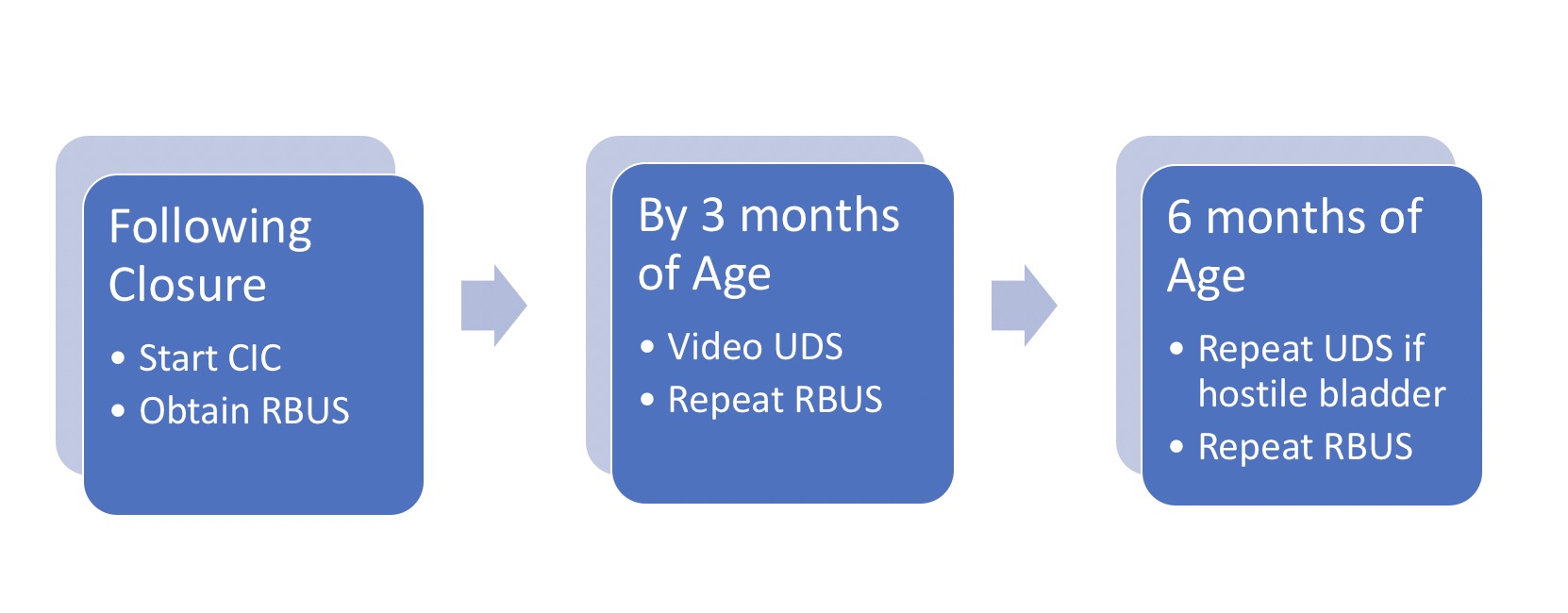
图 2 脊柱裂婴儿初始管理方案。请注意,所有新生儿均按方案每6小时进行CIC;若残余尿量持续≤30 mL且RBUS肾积水≤2级,可逐步延长导尿间隔,随后停止。 *改编自。21
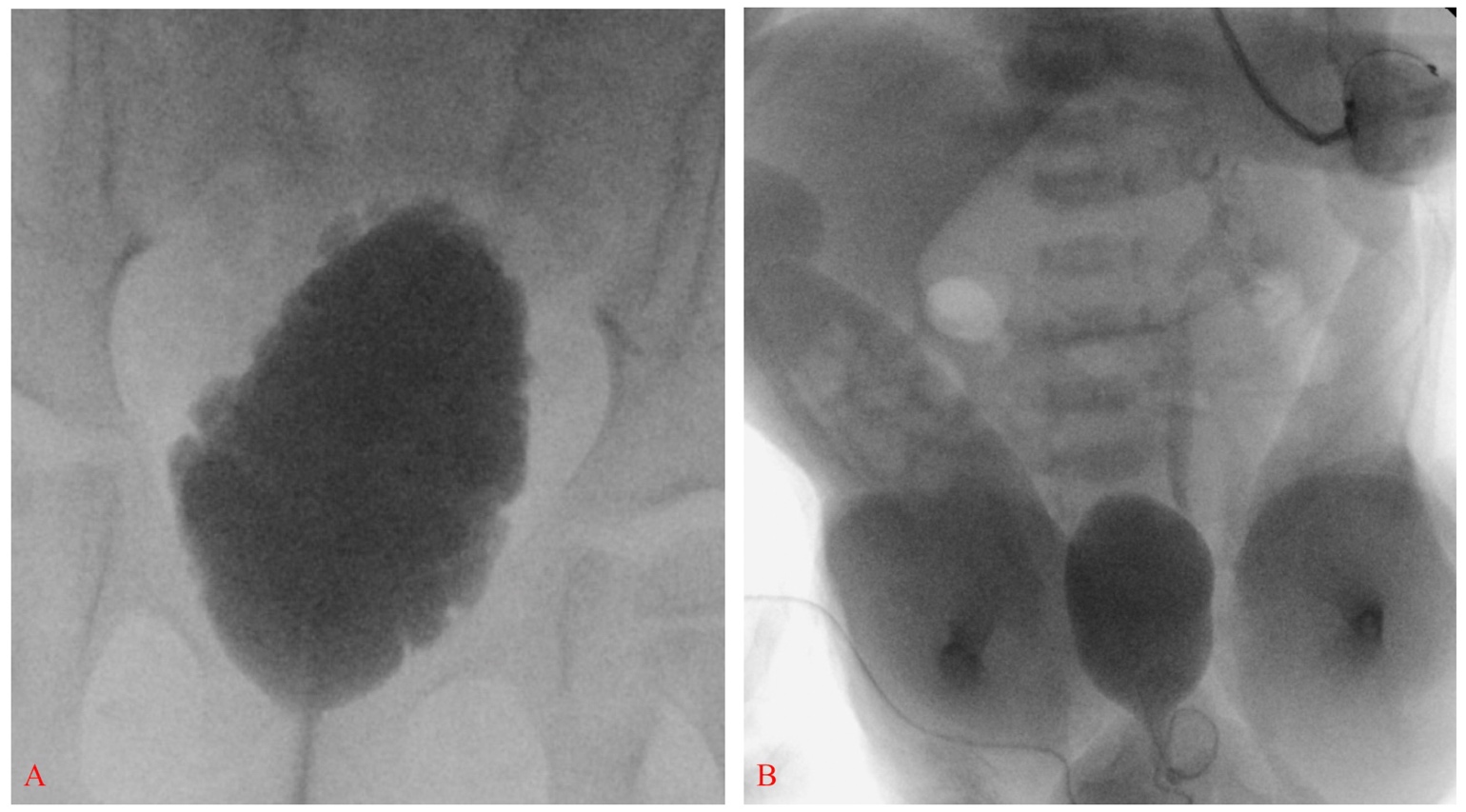
图 3 膀胱延长,轮廓不规则并可见小梁形成,与神经源性膀胱相符(A)。膀胱壁光滑的脊柱裂新生儿伴左侧III级膀胱输尿管返流;另见双侧马蹄内翻足和严重的髋关节屈曲(B)。
治疗方案及其结局
小儿神经源性膀胱的管理目标包括在保护上尿路功能的同时,实现低压储尿并维持控尿能力。对于具有恶化可能性的患儿,当采用期待而非预防性治疗进行随访时,尿路恶化的发生率显著,报道为超过50%。20,23,24 膀胱功能障碍的严重程度差异很大,且随时间而变化,早期尿动力学评估是识别处于较高风险患儿的关键 – 对于具有敌对性参数的个体,建议采取更积极的泌尿外科管理。27,28,29
尽早实施CIC和抗胆碱能治疗已被证明随着时间推移具有优势。间歇性导尿通常每6小时开始一次;对于尿动力学检查(UDS)结果不佳者,清醒时将频率增加至每4小时一次,并加用奥昔布宁 0.2 mg/kg/8 小时。在逼尿肌顺应性低、因此面临肾功能受损风险的患儿中,抗胆碱能药物是治疗逼尿肌过度活动并降低膀胱贮尿压力的主要手段。30,31 除奥昔布宁外,还有数种较新的选择性抗胆碱能药物(如索利那新和托特罗定),其设计旨在通过靶向特定的毒蕈碱受体亚型,或通过改变化学结构使其不太可能跨越器官屏障,从而减少副作用。32 米拉贝隆,一种β3‐肾上腺素能受体激动剂,是抗毒蕈碱药物的替代治疗选择,并已被证明对患有神经源性膀胱功能障碍的儿童和青少年有效且耐受性良好。33 在充分引流并控制逼尿肌过度活动的情况下,膀胱往往保持良好顺应性,随着儿童生长而扩张,并维持适当的壁厚。肾积水和膀胱输尿管反流的发生率低于10%,且多数患儿无需额外干预即可较易实现社会性控尿。20,34,35
A型肉毒毒素(BTX-A)可抑制神经肌肉接头处乙酰胆碱的释放,被认为是改善儿童神经源性膀胱功能障碍的控尿能力和尿动力学参数的一种替代治疗选择。31 研究已显示临床和尿动力学参数显著改善,其证据包括:约65%~87%的患儿达到完全控尿,且在大多数接受治疗者中,逼尿肌最大压力降低、逼尿肌顺应性增加。36
使用小肠或结肠进行膀胱扩大成形术是在大量对内科治疗无效的儿童中,建立低压且大容量储尿器官的最终方法,但其应用可能受短期和长期并发症的限制。37,38,39 可根据指征同时行膀胱颈部手术。手术干预应根据尿动力学结果、病史、年龄、手部灵巧度以及社会因素进行个体化。临床医生必须合理预期患儿家庭已准备好承担术后导尿和冲洗方案。至少三分之一接受膀胱扩大成形术的儿童会发生并发症,包括膀胱穿孔、结石形成,以及需要追加如造口修整等手术。40,41,42 将肠道并入泌尿道的已知后果之一是代谢异常,必须监测包括慢性酸中毒在内的代谢失衡。43 虽然罕见,但恶性肿瘤是扩大术的严重远期并发症,强调了对这些患者进行终身随访的重要性。44,45,46 值得注意的是,与接受期待性随访的儿童相比,为维持足够储尿容量而需要行膀胱扩大成形术的比例显著降低,从近60%降至16%。47
建议的随访
- 应在出生时进行肾膀胱超声检查,生命的第一年每3个月一次,之后每年一次,并同时检测血清肌酐、进行体格检查和血压评估。21
- 3个月时进行基线尿动力学检查;在1、2、3岁每年检查一次,随后在儿童期每隔一年检查一次(若存在敌对膀胱参数、需要评估治疗反应,或出现新的输尿管肾盂积水/尿失禁/反复尿路感染,则需更频繁进行UDS)。21,48
- 随着脊柱裂人群年龄增长,必须让患者、照护者和初级保健医师意识到神经源性膀胱所伴随的基础癌症风险,并且即使未进行会增加泌尿道肿瘤风险的重建或分流手术,也需要密切监测。46
结论
神经源性膀胱涵盖了广泛的疾病谱,其表现取决于下尿路受累程度以及膀胱储尿能力与括约肌功能之间的相互作用。目前神经源性膀胱治疗的基石是非手术治疗,主要包括抗胆碱能药物和清洁间歇导尿。对脊柱裂患儿的积极治疗已被证明可以通过尽量减少高压返流对上尿路的影响,从而降低手术干预的需要并减少终末期肾病(ESRD)的发生。在有明确指征时,手术治疗需个体化制定,基于对尿动力学结果、病史、年龄及是否合并其他残疾的全面考量。
关键要点
- 主动管理可减少神经源性膀胱的长期后遗症
- 逼尿肌充盈期压力升高、膀胱-括约肌失协调或漏点压力升高 (>40 cm H20) 可导致上尿路损害
- 药物治疗与CIC是管理的基石
建议阅读
- Bauer SB. Neurogenic bladder: etiology and assessment. Pediatr Nephrol 2008; 23 (4): 541–551. DOI: 10.1007/s00467-008-0764-7.
- Routh JC, Cheng EY, Austin JC, Baum MA, Gargollo PC, Grady RW, et al.. Design and Methodological Considerations of the Centers for Disease Control and Prevention Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida. J Urol 2016; 196 (6): 1728–1734. DOI: 10.1016/j.juro.2016.07.081.
- Rawashdeh YF, Austin P, Siggaard C, Bauer SB, Franco I, Jong TP de, et al.. International children’s continence society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn 2012; 31 (5): 615–620. DOI: 10.1002/nau.22248.
参考文献
- Bauer SB. Neurogenic bladder: etiology and assessment. Pediatr Nephrol 2008; 23 (4): 541–551. DOI: 10.1007/s00467-008-0764-7.
- OAKESHOTT PIPPA, HUNT GILLIANM, POULTON ALISON, REID FIONA. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol 2010; 52 (8): 749–753. DOI: 10.1111/j.1469-8749.2009.03543.x.
- Hadžagić-Ćatibušić F, Maksić H, Užičanin S, Heljić S, Zubčević S, Merhemić Z, et al.. Congenital Malformations of the Central Nervous System: Clinical Approach. Bosn J Basic Med Sci 2008; 8 (4): 356–360. DOI: 10.17305/bjbms.2008.2897.
- Blaivas JG, Labib KL, Bauer SB, Retik AB. Changing Concepts in the Urodynamic Evaluation of Children. J Urol 1977; 117 (6): 778–781. DOI: 10.1016/s0022-5347(17)58623-1.
- Northrup H, Volcik KA. Spina bifida and other neural tube defects. Curr Probl Pediatr 2000; 30 (10): 317–332. DOI: 10.1067/mpp.2000.112052.
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al.. Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol 2010; 88 (12): 1008–1016. DOI: 10.1002/bdra.20735.
- Ryznychuk MO, Kryvchanska MI, Lastivka IV. Teaching children with spina bifida. The Child with Spina Bifida 2018; 71 (339-344): 202–236. DOI: 10.4324/9781315656861-9.
- Honein MA, Paulozzi LJ, Mathews TJ. Impact of Folic Acid Fortification of the US Food Supply on the Occurrence of Neural Tube Defects–Correction. Jama 2001; 286 (18): 2236. DOI: 10.1001/jama.286.18.2236.
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the Prevalence of Spina Bifida and Anencephaly by Race/Ethnicity: 1995–2002. Pediatrics 2005; 116 (3): 580–586. DOI: 10.1542/peds.2005-0592.
- Bestwick JP, Huttly WJ, Morris JK, Wald NJ. Prevention of Neural Tube Defects: A Cross-Sectional Study of the Uptake of Folic Acid Supplementation in Nearly Half a Million Women. PLoS One 2014; 9 (2): e89354. DOI: 10.1371/journal.pone.0089354.
- Agopian AJ, Canfield MA, Olney RS, Lupo PJ, Ramadhani T, Mitchell LE, et al.. Spina bifida subtypes and sub-phenotypes by maternal race/ethnicity in the National Birth Defects Prevention Study. Am J Med Genet A 2012; 158a (1): 109–115. DOI: 10.1002/ajmg.a.34383.
- Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet 2004; 364 (9448): 1885–1895. DOI: 10.1016/s0140-6736(04)17445-x.
- Jobe AH. Fetal Surgery for Myelomeningocele. N Engl J Med 2002; 347 (4): 230–231. DOI: 10.1056/nejmp020073.
- Mohd-Zin SW, Marwan AI, Abou Chaar MK, Ahmad-Annuar A, Abdul-Aziz NM. Spina Bifida: Pathogenesis, Mechanisms, and Genes in Mice and Humans. Scientifica (Cairo) 2017; 2017: 1–29. DOI: 10.1155/2017/5364827.
- Stevenson KL. Chiari Type II malformation: past, present, and future. Neurosurg Focus 2004; 16 (2): 1–7. DOI: 10.3171/foc.2004.16.2.6.
- Adzick NS, Thom EA, Spong CY. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. Yearbook of Pediatrics 2011; 2012 (11): 404–406. DOI: 10.1016/j.yped.2011.06.029.
- Wang H-HS, Lloyd JC, Wiener JS, Routh JC. Nationwide Trends and Variations in Urological Surgical Interventions and Renal Outcome in Patients with Spina Bifida. J Urol 2016; 195 (4 Part 2): 1189–1195. DOI: 10.1016/j.juro.2015.11.033.
- KURZROCK ERICA, POLSE SANFORD. Renal Deterioration In Myelodysplastic Children: Urodynamic Evaluation And Clinical Correlates. J Urol 1998; 159 (5): 1657–1661. DOI: 10.1097/00005392-199805000-00084.
- HOPPS CARINV, KROPP KENNETHA. Preservation of Renal Function in Children With Myelomeningocele Managed With Basic Newborn Evaluation And Close Followup. J Urol 2003; 169: 305–308. DOI: 10.1097/00005392-200301000-00092.
- Wu H-Y, Baskin L, Kogan BA. Neurogenic Bladder Dysfunction Due to Myelomeningocele: Neonatal Versus Childhood Treatment. J Urol 1997; 157 (6): 2295–2297. DOI: 10.1016/s0022-5347(01)64766-9.
- Routh JC, Cheng EY, Austin JC, Baum MA, Gargollo PC, Grady RW, et al.. Design and Methodological Considerations of the Centers for Disease Control and Prevention Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida. J Urol 2016; 196 (6): 1728–1734. DOI: 10.1016/j.juro.2016.07.081.
- Mcguire EJ, Wang C-C, Usitalo H, Savastano J. Modified Pubovaginal Sling in Girls with Myelodysplasia. J Urol 1986; 135 (1): 94–96. DOI: 10.1016/s0022-5347(17)45528-5.
- Bauer SB, Hallet M, Khoshbin S. Predictive value of urodynamic evaluation in newborns with myelodysplasia. Jama 1984; 252 (5): 650–652. DOI: 10.1001/jama.252.5.650.
- Ami Sidi A, Dykstra DD, Gonzalez R. The Value of Urodynamic Testing in the Management of Neonates with Myelodysplasia: A Prospective Study. J Urol 1986; 135 (1): 90–93. DOI: 10.1016/s0022-5347(17)45527-3.
- Perez LM, Khoury J, Webster GD. The Value of Urodynamic Studies in Infants Less than 1 Year Old with Congenital Spinal Dysraphism. J Urol 1992; 148 (2 Part 2): 584–587. DOI: 10.1016/s0022-5347(17)36660-0.
- BASKIN LS, KOGAN BA, BENARD F. Treatment of Infants with Neurogenic Bladder Dysfunction using Anticholinergic Drugs and Intermittent Catheterisation. Br J Urol 1990; 66 (5): 532–534. DOI: 10.1111/j.1464-410x.1990.tb15004.x.
- Joseph DB, Bauer SB, Colodny AH, Mandell J, Retik AB. Clean, Intermittent Catheterization of Infants With Neurogenic Bladder. Pediatrics 1989; 84 (1): 78–82. DOI: 10.1542/peds.84.1.78.
- Tanaka ST, Yerkes EB, Routh JC, Tu DD, Austin JC, Wiener JS, et al.. Urodynamic characteristics of neurogenic bladder in newborns with myelomeningocele and refinement of the definition of bladder hostility: Findings from the UMPIRE multi-center study. J Pediatr Urol 2021; 17 (5): 726–732. DOI: 10.1016/j.jpurol.2021.04.019.
- Snow-Lisy DC, Yerkes EB, Cheng EY. Update on Urological Management of Spina Bifida from Prenatal Diagnosis to Adulthood. J Urol 2015; 194 (2): 288–296. DOI: 10.1016/j.juro.2015.03.107.
- Ghoniem GM, Roach MB, Lewis VH, Harmon EP. The Value of Leak Pressure and Bladder Compliance in the Urodynamic Evaluation of Meningomyelocele Patients. J Urol 1990; 144 (6): 1440–1442. DOI: 10.1016/s0022-5347(17)39761-6.
- Galloway NTM, Mekras JA, Helms M, Webster GD. An Objective Score to Predict Upper Tract Deterioration in Myelodysplasia. J Urol 1991; 145 (3): 535–537. DOI: 10.1016/s0022-5347(17)38389-1.
- Andersson K-E, Chapple CR, Cardozo L, Cruz F, Hashim H, Michel MC, et al.. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol 2009; 19 (4): 380–394. DOI: 10.1097/mou.0b013e32832ce8a4.
- Rawashdeh YF, Austin P, Siggaard C, Bauer SB, Franco I, Jong TP de, et al.. International children’s continence society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn 2012; 31 (5): 615–620. DOI: 10.1002/nau.22248.
- Franco I, Hoebeke P. Baka-Ostrowska M, et al. Long-term efficacy and safety of solifenacin in pediatric patients aged 6 months to 18 years with neurogenic detrusor overactivity: results from two phase 3 prospective open-label studies. J Pediatr Urol 2019. 6 (2): 80 1–180 8. DOI: 10.1016/j.jpurol.2019.12.012.
- REINBERG Y, CROCKER J, WOLPERT J, VANDERSTEEN D. Therapeutic Efficacy Of Extended Release Oxybutynin Chloride, And Immediate Release And Long Acting Tolterodine Tartrate In Children With Diurnal Urinary Incontinence. J Urol 2003; 169 (317-319): 317–319. DOI: 10.1097/00005392-200301000-00095.
- Baka-Ostrowska M, Bolong DT, Persu C, Tøndel C, Steup A, Lademacher C, et al.. Efficacy and safety of mirabegron in children and adolescents with neurogenic detrusor overactivity: An open-label, phase 3, dose-titration study. Neurourol Urodyn 2021; 40 (6): 1490–1499. DOI: 10.1002/nau.24657.
- Edelstein RA, Bauer SB, Kelly MD. The long-term urologic response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Pediatr Surg 1995; 31 (3): 455–456. DOI: 10.1016/s0022-3468(96)90810-6.
- Karsenty G, Denys P, Amarenco G, De Seze M, Gamé X, Haab F, et al.. Botulinum Toxin A (Botox®) Intradetrusor Injections in Adults with Neurogenic Detrusor Overactivity/Neurogenic Overactive Bladder: A Systematic Literature Review. Eur Urol 2009; 53 (2): 275–287. DOI: 10.1016/j.eururo.2007.10.013.
- Lemelle JL, Guillemin F, Aubert D, Guys JM, Lottmann H, Lortat-Jacob S, et al.. A Multicenter Evaluation of Urinary Incontinence Management and Outcome in Spina Bifida. J Urol 2006; 175 (1): 208???212. DOI: 10.1097/00005392-200601000-00056.
- Metcalfe PD, Rink RC. Bladder augmentation: Complications in the pediatric population. Curr Urol Rep 2007; 8 (2): 152–156. DOI: 10.1007/s11934-007-0065-x.
- Walker RD. The management of the failed bladder neck procedure in patients with spina bifida. BJU Int 2016; 92: 35–37. DOI: 10.1046/j.1464-410x.92.s1.13.x.
- Scales CD, Wiener JS. Evaluating Outcomes of Enterocystoplasty in Patients With Spina Bifida: A Review of the Literature. J Urol 2008; 180 (6): 2323–2329. DOI: 10.1016/j.juro.2008.08.050.
- Kurzrock EA. Pediatric enterocystoplasty: long-term complications and controversies. World J Urol 2009; 27 (1): 69–73. DOI: 10.1007/s00345-008-0335-3.
- Merriman LS, Arlen AM, Kirsch AJ, Leong T, Smith EA. Does augmentation cystoplasty with continent reconstruction at a young age increase the risk of complications or secondary surgeries? J Pediatr Urol 2015; 11 (1): 41.e1–41.e5. DOI: 10.1016/j.jpurol.2014.08.016.
- Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep 2007; 8 (2): 157–162. DOI: 10.1007/s11934-007-0066-9.
- Husmann DA. Malignancy after gastrointestinal augmentation in childhood. Ther Adv Urol 2009; 1 (1): 5–11. DOI: 10.1177/1756287209104163.
- Austin JC. Long-term risks of bladder augmentation in pediatric patients. Curr Opin Urol 2008; 18 (4): 408–412. DOI: 10.1097/mou.0b013e328300587c.
- Arlen AM, Dudley AG, Kieran K. Association of spina bifida with cancer. Transl Androl Urol 2020; 9 (5): 2358–2369. DOI: 10.21037/tau-19-771.
- Kaefer M, Pabby A, Kelly M. Improved Bladder Function After Prophylactic Treatment Of The High Risk Neurogenic Bladder In Newborns With Myelomeningocele. J Urol 1999; 162: 1071. DOI: 10.1097/00005392-199909000-00032.
- Bauer SB, Nijman RJM, Drzewiecki BA, Sillen U, Hoebeke P. International Children’s Continence Society standardization report on urodynamic studies of the lower urinary tract in children. Neurourol Urodyn 2015; 34 (7): 640–647. DOI: 10.1002/nau.22783.
最近更新时间: 2025-09-22 08:00