19: Neurogenic Bladder
Este capítulo durará aproximadamente 12 minutos para leer.
Introduction
Congenital spinal dysraphism accounts for the vast majority of cases of pediatric neurogenic bladder and bowel.1 Other causes of neurogenic dysfunction involving the spine include sacral agenesis, tethered cord, cloacal malformations, and spinal cord injuries. Children with central nervous system abnormalities such as spastic diplegia (cerebral palsy) may also experience neurogenic bladder.
Poor bladder dynamics can lead to chronic kidney disease and a multitude of potential sequelae.2 Management of neurogenic bladder includes achieving low pressure urinary storage and providing urinary continence while preserving upper tract function. Optimal treatment requires a multidisciplinary care team to prevent and manage potential sequelae that may affect function, quality of life, and survival. Given that spina bifida accounts for most cases of neurogenic bladder, it will be the focus of the chapter herein.
Embryology
Neural tube defects (NTDs) are malformations of the brain and spinal cord resulting from failure of the neural tube to close during the third and fourth weeks of intrauterine development.3 Spina bifida occurs when the spinal cord is exposed and/or protrudes to the surface with the meninges through a vertebral wall defect. Spinal canal development begins on the 18th day of gestation and is completed by day 35, closing in a caudad direction from the cephalic end of the body. Spinal dysraphism is attributable to abnormal development of the cranial mesoderm.3 Failure of mesodermal ingrowth over the developing spinal cord results in an open lesion, most commonly seen in the lumbosacral area (Table 1). The exposed spinal cord and its nerve roots, which may protrude into the meningocele sac, and tension on the spinal cord as it moves cranially up the canal with elongation of the fetus (from L2, L3 in mid-late gestation, to L1 at birth), contribute to the variable picture of neural injury to the lower urinary tract and lower extremities.4 Hydrocephalus and Arnold-Chiari malformation type II (a combination of myelomeningocele and cerebellar tonsil herniation) are commonly associated with spina bifida.5
Table 1 Spinal level of myelomeningocele.
| Location | Incidence |
|---|---|
| Cervical-High Thoracic | 2% |
| Low Thoracic | 5% |
| Lumbar | 26% |
| Lumbosacral | 47% |
| Sacral | 20% |
Epidemiology
Spina bifida is the most common neural tube defect, occurring in approximately 1 out of every 3,000 live births in the United States and 1 per 1,000 live births worldwide.6,7 Although the incidence of spina bifida significantly decreased after introduction of mandatory fortification of enriched grain products with folic acid in the United States, the population burden of spina bifida continues both in birth prevalence and in disparate long-term outcomes.8,9,10 Differences in maternal race/ethnicity have been shown to impact the prevalence of spina bifida with Hispanic mothers having a higher prevalence than Caucasian and Black women.11 Gender preponderance differs according to country; in the USA, spina bifida is thought to be more prevalent in girls than boys.12
Pathogenesis
Spina bifida is typically visible at birth as exposed neural tissue with or without a protruding sac at the site of the lesion. Myelomeningocele is when the spinal cord protrudes from the spinal canal into a fluid-filled sac resulting from incomplete closure of the primary neural tube (Figure 1) The extent and severity of the neurological deficits depend on the location of the lesion along the neuraxis - depending on the level, interruption of the spinal cord at the site of the defect causes paralysis of the legs, incontinence of urine and feces, anesthesia of the skin, and abnormalities of the hips, knees, and feet.13,14 There is variable impact on somatic, parasympathetic and sympathetic innervation of the detrusor and sphincteric muscles, that affects the bladder’s ability to store and empty urine - myelomeningocele is almost always associated with neurogenic bladder. Poor bladder dynamics can then lead to chronic kidney disease and a multitude of potential sequelae.2
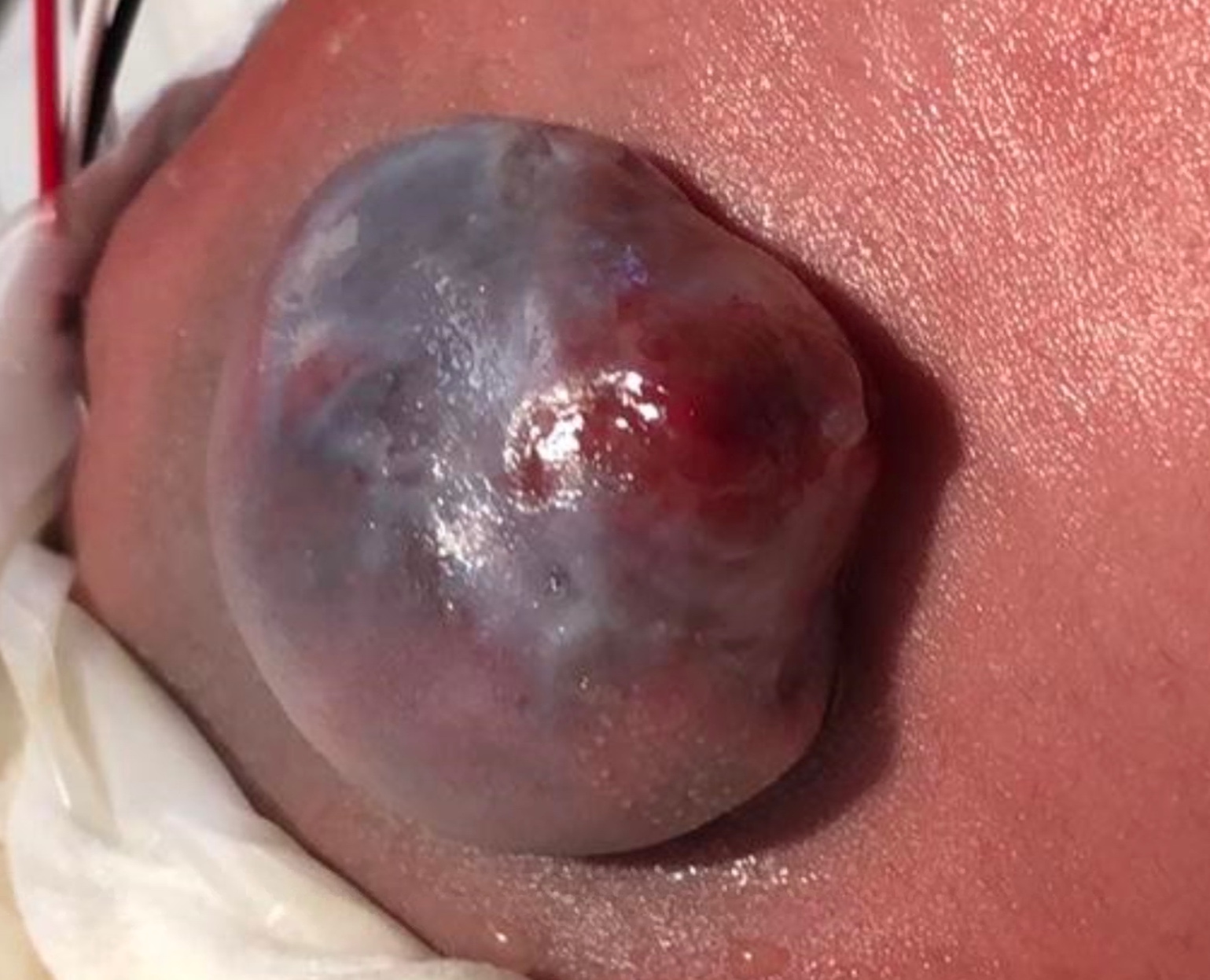
Figure 1 Open myelomeningocele.
Myelomeningocele is usually associated with a type II Chiari hindbrain malformation, ventriculomegaly, and hydrocephalus.15 Chiari type II malformation is the downward displacement of the cerebellar vermis into the cervical vertebral canal.5 It results in elongation of the brain stem and obliteration of the fourth ventricle, leading to obstruction of cerebrospinal fluid circulation and development of hydrocephalus in 90% of patients.5,13 Treatment of hydrocephalus is required in approximately 82% of cases and involves draining of cerebrospinal fluid into either the peritoneal or other body cavity via a shunt.16
Evaluation and Diagnosis
While optimal care of spina bifida related bladder dysfunction remains unknown, there has been a recent trend toward proactive rather than reactive management of children with neurogenic bladder.17 Observation involves periodic monitoring with noninvasive testing, including renal-bladder ultrasound, to evaluate for upper tract dilation/deterioration. However, many pediatric urologists now advocate for a full investigation of the lower urinary tract in infancy with initiation of prophylactic treatment either at birth or if there are signs of outlet obstruction and/or elevated bladder filling or voiding pressure on initial urodynamic testing.18,19,20
In 2012, the Centers for Disease Control and Prevention (CDC) convened a working group of pediatric urologists, nephrologists, clinical epidemiologists, methodologists, community advocates and CDC personnel to develop a standardized protocol to optimize urological care of children with spina bifida from the newborn period through age 5 (Figure 2)21 Renal-bladder ultrasound is obtained to determine baseline upper tract dilation.17 Videourodynamics are obtained by 3 months of age – fluoroscopic images demonstrate vesicoureteral reflux if present as well as irregular bladder contour/trabeculation (Figure 3)]. The prevailing issue at birth is whether detrusor sphincter dyssynergia is present and if the baby can empty at low pressures. Elevated detrusor filling pressure, bladder sphincter dyssynergia or elevated leak point pressures (>40 cm H20) can result in upper tract damage and warrants early intervention with clean intermittent catheterization (CIC) and anticholinergics.22,23,24,25 With a proactive approach, infants are placed on scheduled clean intermittent catheterization (CIC) every 6 hours – the frequency of catheterizations may be decreased if volumes are consistently ≤30 mL; a key component is family learning to perform catheterizations prior to discharge.21,26
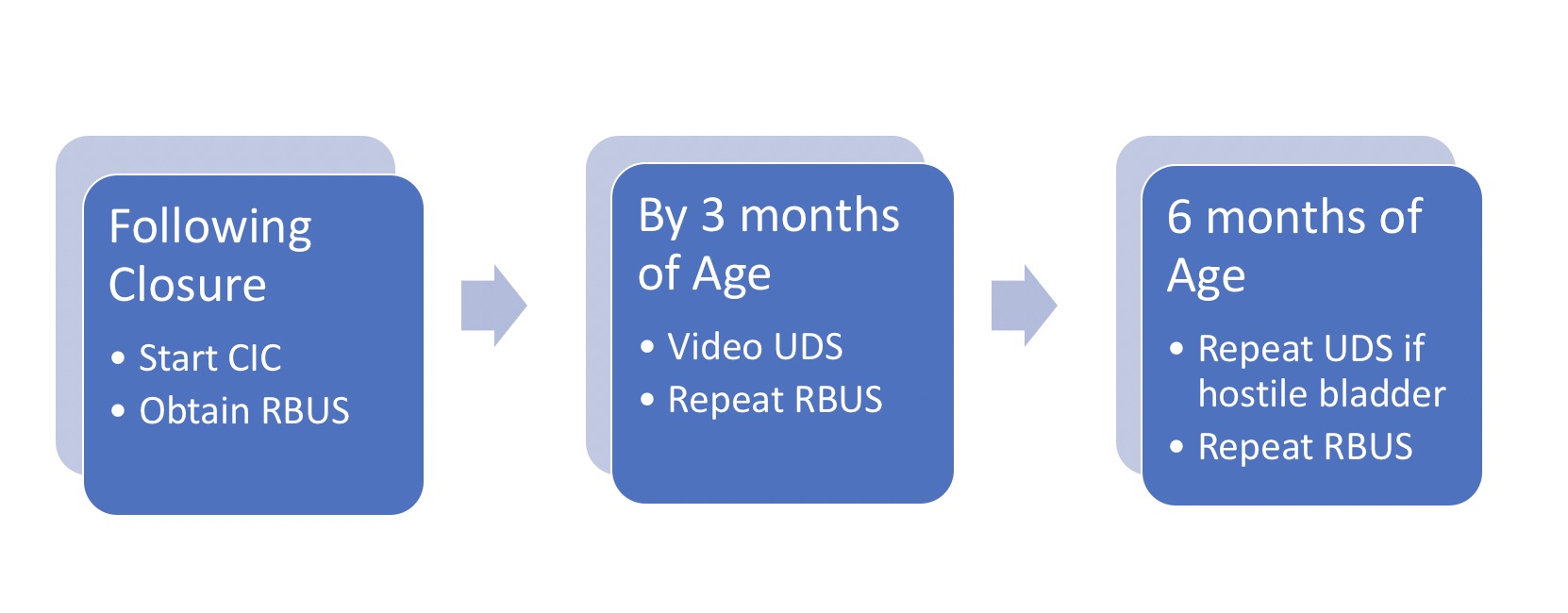
Figure 2 Protocol for initial management of infant with spina bifida. Note that all newborns are started on CIC every 6 hours per protocol; catheterization interval may be increased and then discontinued if residuals consistently ≤30 mL and RBUS hydronephrosis ≤ grade 2. *Adapted from.21
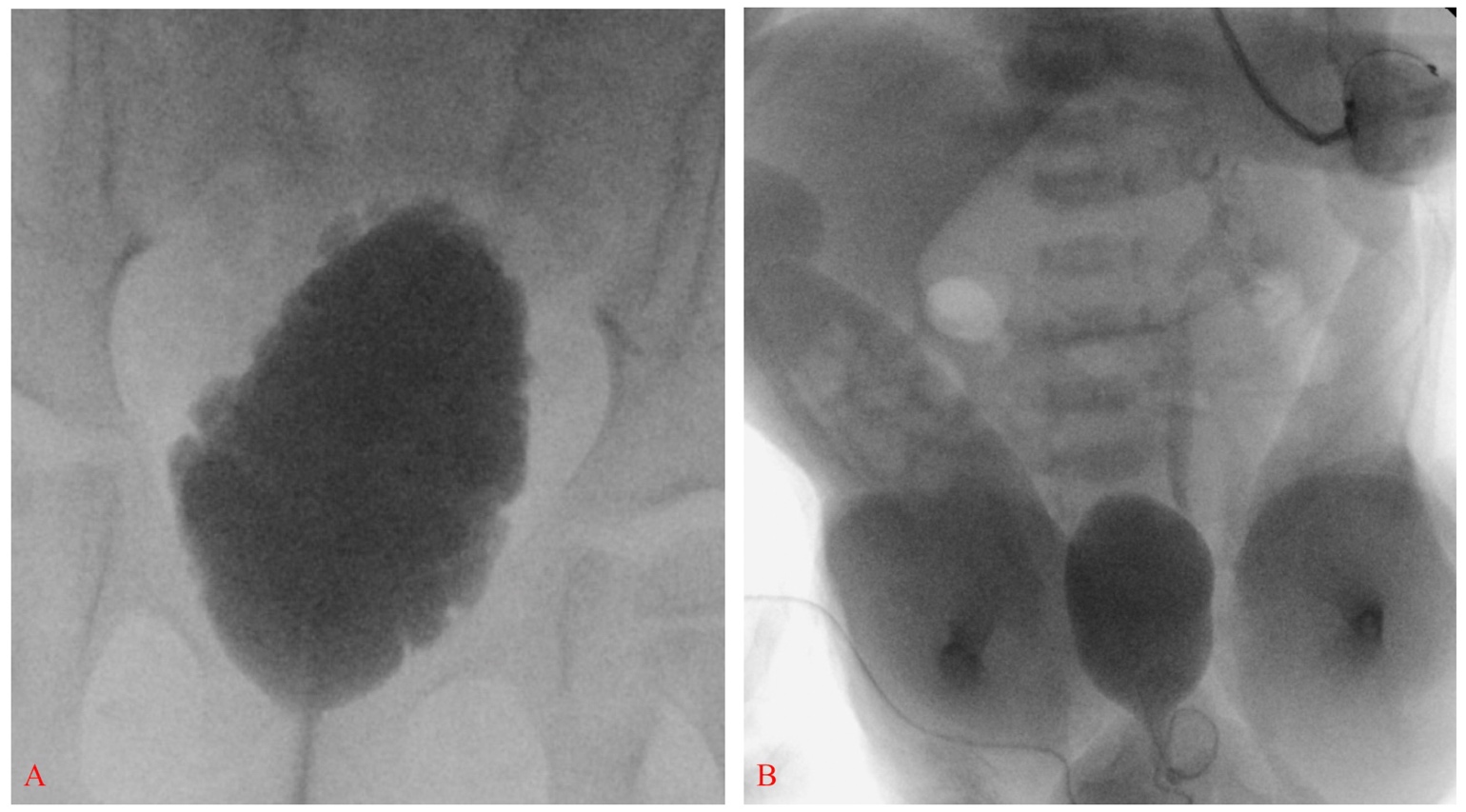
Figure 3 Elongated bladder with irregular contour and trabeculation consistent with neurogenic bladder (A). Smooth-walled bladder with left grade III vesicoureteral reflux in a newborn with spina bifida; also note bilateral clubfeet and severe hip flexion (B).
Treatment Options and Their Outcomes
The goals of management of pediatric neurogenic bladder include achieving low pressure urinary storage and providing urinary continence while preserving upper tract function. The incidence of urinary tract deterioration is significant, reported at greater than 50%, when children with the potential for deterioration are followed with expectant rather than preemptive therapy.20,23,24 Severity of bladder dysfunction varies widely and changes over time, early urodynamic evaluation is key identifying children at increased risk – more aggressive urologic management is recommended for those individuals with hostile parameters.27,28,29
Early implementation of CIC and anticholinergic therapy has proven advantageous over time. Intermittent catheterization is typically initiated every 6 hours, with frequency increased to every 4 hours while awake and addition of oxybutynin 0.2 mg/kg/8 hours in those with unfavorable UDS findings. Anticholinergics are the mainstay to treat detrusor overactivity and reduce intravesical storage pressures in children with low detrusor compliance that places them at risk for renal compromise.30,31 In addition to oxybutynin, there are several newer selective anticholinergic medications (such as solifenacin and tolterodine) that are designed to diminish side effects by either targeting specific muscarinic receptor subtypes or by altering the structural compounds so that they are less likely to cross organ barriers.32 Mirabegron, a β3‐adrenoreceptor agonist, is an alternative treatment option to antimuscarinics, and has been shown to be effective and well-tolerated in children and adolescents with neurogenic bladder dysfunction.33 With adequate drainage and management of detrusor overactivity, the bladder often remains compliant, expanding as the child grows, and maintaining appropriate wall thickness. Hydronephrosis and vesicoureteral reflux develop in fewer than 10% and social continence is readily achieved in the majority of patients with no additional inventions.20,34,35
Botulinum-A Toxin (BTX-A) inhibits acetylcholine neurotransmitter release at the neuromuscular junction and is considered an alternative to improving continence and urodynamic parameters of neurogenic bladder dysfunction in children.31 Studies have demonstrated significant improvement in clinical and urodynamic parameters as evidenced by complete continence in approximately 65% to 87% of children and a reduction in maximum detrusor pressure and an increase in detrusor compliance in the majority of those treated.36
Bladder augmentation using small intestine or colon represents the definitive method of creating a low-pressure capacious organ for urine storage in a substantial number of children who have failed medical management, but its utility can be limited by both short- and long-term morbidity.37,38,39 Concomitant bladder neck procedures are performed as indicated. Surgical intervention should be individualized based on urodynamic findings, medical history, age, manual dexterity as well as social factors. The clinician must have a reasonable expectation that the family is prepared to undertake post-operative catheterization and irrigation regimens. At least one-third of children undergoing bladder augmentation will experience a complication, including bladder perforation, stone formation and need for additional procedures such as stomal revision.40,41,42 Metabolic abnormalities are a known consequence of incorporating bowel into the urinary tract, and patients must be monitored for metabolic imbalances including chronic acidosis.43 Malignancy, while rare, is a serious late complication of augmentation and underscores the importance of lifelong follow up for these patients.44,45,46 It is worth noting the need for augmentation cystoplasty to maintain adequate storage capacity is markedly reduced from almost 60% to 16% when compared to children followed expectantly.47
Suggested Follow-Up
- Renal-bladder ultrasound should be obtained at birth, every 3 months in the first year of life, and then annually along with serum creatinine, physical exam and blood pressure assessment.21
- Baseline urodynamics at 3 months; annual study age 1, 2 and 3 years then every other year during childhood (UDS are obtained more frequently if hostile parameters, assessing response to therapy or new hydroureteronephrosis/incontinence/recurrent urinary tract infection).21,48
- As the spina bifida population ages, patients, caregivers, and primary care physicians must be made aware of the baseline risk of cancer with neurogenic bladder and the need for close monitoring even in the absence of reconstructive and diverting procedures which increase the neoplastic risk in the urinary tract.46
Conclusions
Neurogenic bladder encompasses a wide spectrum of disease depending on the degree of lower urinary tract involvement and the interplay between bladder storage capability and sphincter function. The mainstay of current neurogenic bladder management is non-surgical with anticholinergics and clean intermittent catheterization. Proactive treatment of children with spina bifida has been shown to be effective in reducing the need for surgical intervention and the development of ESRD by minimizing the effects of high-pressure reflux on the upper urinary tract. When indicated, surgical management is tailored to each individual patient, based on careful consideration of urodynamic findings, medical history, age, and presence of other disability.
Key Points
- Proactive management reduces long-term sequelae of neurogenic bladder
- Elevated detrusor filling pressure, bladder sphincter dyssynergia or elevated leak point pressures (>40 cm H20) can result in upper tract damage
- Pharmacotherapy and CIC are the cornerstones of management
Suggested Readings
- Bauer SB. Neurogenic bladder: etiology and assessment. Pediatr Nephrol 2008; 23 (4): 541–551. DOI: 10.1007/s00467-008-0764-7.
- Routh JC, Cheng EY, Austin JC, Baum MA, Gargollo PC, Grady RW, et al.. Design and Methodological Considerations of the Centers for Disease Control and Prevention Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida. J Urol 2016; 196 (6): 1728–1734. DOI: 10.1016/j.juro.2016.07.081.
- Rawashdeh YF, Austin P, Siggaard C, Bauer SB, Franco I, Jong TP de, et al.. International children’s continence society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn 2012; 31 (5): 615–620. DOI: 10.1002/nau.22248.
References
- Bauer SB. Neurogenic bladder: etiology and assessment. Pediatr Nephrol 2008; 23 (4): 541–551. DOI: 10.1007/s00467-008-0764-7.
- OAKESHOTT PIPPA, HUNT GILLIANM, POULTON ALISON, REID FIONA. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev Med Child Neurol 2010; 52 (8): 749–753. DOI: 10.1111/j.1469-8749.2009.03543.x.
- Hadžagić-Ćatibušić F, Maksić H, Užičanin S, Heljić S, Zubčević S, Merhemić Z, et al.. Congenital Malformations of the Central Nervous System: Clinical Approach. Bosn J Basic Med Sci 2008; 8 (4): 356–360. DOI: 10.17305/bjbms.2008.2897.
- Blaivas JG, Labib KL, Bauer SB, Retik AB. Changing Concepts in the Urodynamic Evaluation of Children. J Urol 1977; 117 (6): 778–781. DOI: 10.1016/s0022-5347(17)58623-1.
- Northrup H, Volcik KA. Spina bifida and other neural tube defects. Curr Probl Pediatr 2000; 30 (10): 317–332. DOI: 10.1067/mpp.2000.112052.
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al.. Updated national birth prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol 2010; 88 (12): 1008–1016. DOI: 10.1002/bdra.20735.
- Ryznychuk MO, Kryvchanska MI, Lastivka IV. Teaching children with spina bifida. The Child with Spina Bifida 2018; 71 (339-344): 202–236. DOI: 10.4324/9781315656861-9.
- Honein MA, Paulozzi LJ, Mathews TJ. Impact of Folic Acid Fortification of the US Food Supply on the Occurrence of Neural Tube Defects–Correction. Jama 2001; 286 (18): 2236. DOI: 10.1001/jama.286.18.2236.
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the Prevalence of Spina Bifida and Anencephaly by Race/Ethnicity: 1995–2002. Pediatrics 2005; 116 (3): 580–586. DOI: 10.1542/peds.2005-0592.
- Bestwick JP, Huttly WJ, Morris JK, Wald NJ. Prevention of Neural Tube Defects: A Cross-Sectional Study of the Uptake of Folic Acid Supplementation in Nearly Half a Million Women. PLoS One 2014; 9 (2): e89354. DOI: 10.1371/journal.pone.0089354.
- Agopian AJ, Canfield MA, Olney RS, Lupo PJ, Ramadhani T, Mitchell LE, et al.. Spina bifida subtypes and sub-phenotypes by maternal race/ethnicity in the National Birth Defects Prevention Study. Am J Med Genet A 2012; 158a (1): 109–115. DOI: 10.1002/ajmg.a.34383.
- Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet 2004; 364 (9448): 1885–1895. DOI: 10.1016/s0140-6736(04)17445-x.
- Jobe AH. Fetal Surgery for Myelomeningocele. N Engl J Med 2002; 347 (4): 230–231. DOI: 10.1056/nejmp020073.
- Mohd-Zin SW, Marwan AI, Abou Chaar MK, Ahmad-Annuar A, Abdul-Aziz NM. Spina Bifida: Pathogenesis, Mechanisms, and Genes in Mice and Humans. Scientifica (Cairo) 2017; 2017: 1–29. DOI: 10.1155/2017/5364827.
- Stevenson KL. Chiari Type II malformation: past, present, and future. Neurosurg Focus 2004; 16 (2): 1–7. DOI: 10.3171/foc.2004.16.2.6.
- Adzick NS, Thom EA, Spong CY. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. Yearbook of Pediatrics 2011; 2012 (11): 404–406. DOI: 10.1016/j.yped.2011.06.029.
- Wang H-HS, Lloyd JC, Wiener JS, Routh JC. Nationwide Trends and Variations in Urological Surgical Interventions and Renal Outcome in Patients with Spina Bifida. J Urol 2016; 195 (4 Part 2): 1189–1195. DOI: 10.1016/j.juro.2015.11.033.
- KURZROCK ERICA, POLSE SANFORD. Renal Deterioration In Myelodysplastic Children: Urodynamic Evaluation And Clinical Correlates. J Urol 1998; 159 (5): 1657–1661. DOI: 10.1097/00005392-199805000-00084.
- HOPPS CARINV, KROPP KENNETHA. Preservation of Renal Function in Children With Myelomeningocele Managed With Basic Newborn Evaluation And Close Followup. J Urol 2003; 169: 305–308. DOI: 10.1097/00005392-200301000-00092.
- Wu H-Y, Baskin L, Kogan BA. Neurogenic Bladder Dysfunction Due to Myelomeningocele: Neonatal Versus Childhood Treatment. J Urol 1997; 157 (6): 2295–2297. DOI: 10.1016/s0022-5347(01)64766-9.
- Routh JC, Cheng EY, Austin JC, Baum MA, Gargollo PC, Grady RW, et al.. Design and Methodological Considerations of the Centers for Disease Control and Prevention Urologic and Renal Protocol for the Newborn and Young Child with Spina Bifida. J Urol 2016; 196 (6): 1728–1734. DOI: 10.1016/j.juro.2016.07.081.
- Mcguire EJ, Wang C-C, Usitalo H, Savastano J. Modified Pubovaginal Sling in Girls with Myelodysplasia. J Urol 1986; 135 (1): 94–96. DOI: 10.1016/s0022-5347(17)45528-5.
- Bauer SB, Hallet M, Khoshbin S. Predictive value of urodynamic evaluation in newborns with myelodysplasia. Jama 1984; 252 (5): 650–652. DOI: 10.1001/jama.252.5.650.
- Ami Sidi A, Dykstra DD, Gonzalez R. The Value of Urodynamic Testing in the Management of Neonates with Myelodysplasia: A Prospective Study. J Urol 1986; 135 (1): 90–93. DOI: 10.1016/s0022-5347(17)45527-3.
- Perez LM, Khoury J, Webster GD. The Value of Urodynamic Studies in Infants Less than 1 Year Old with Congenital Spinal Dysraphism. J Urol 1992; 148 (2 Part 2): 584–587. DOI: 10.1016/s0022-5347(17)36660-0.
- BASKIN LS, KOGAN BA, BENARD F. Treatment of Infants with Neurogenic Bladder Dysfunction using Anticholinergic Drugs and Intermittent Catheterisation. Br J Urol 1990; 66 (5): 532–534. DOI: 10.1111/j.1464-410x.1990.tb15004.x.
- Joseph DB, Bauer SB, Colodny AH, Mandell J, Retik AB. Clean, Intermittent Catheterization of Infants With Neurogenic Bladder. Pediatrics 1989; 84 (1): 78–82. DOI: 10.1542/peds.84.1.78.
- Tanaka ST, Yerkes EB, Routh JC, Tu DD, Austin JC, Wiener JS, et al.. Urodynamic characteristics of neurogenic bladder in newborns with myelomeningocele and refinement of the definition of bladder hostility: Findings from the UMPIRE multi-center study. J Pediatr Urol 2021; 17 (5): 726–732. DOI: 10.1016/j.jpurol.2021.04.019.
- Snow-Lisy DC, Yerkes EB, Cheng EY. Update on Urological Management of Spina Bifida from Prenatal Diagnosis to Adulthood. J Urol 2015; 194 (2): 288–296. DOI: 10.1016/j.juro.2015.03.107.
- Ghoniem GM, Roach MB, Lewis VH, Harmon EP. The Value of Leak Pressure and Bladder Compliance in the Urodynamic Evaluation of Meningomyelocele Patients. J Urol 1990; 144 (6): 1440–1442. DOI: 10.1016/s0022-5347(17)39761-6.
- Galloway NTM, Mekras JA, Helms M, Webster GD. An Objective Score to Predict Upper Tract Deterioration in Myelodysplasia. J Urol 1991; 145 (3): 535–537. DOI: 10.1016/s0022-5347(17)38389-1.
- Andersson K-E, Chapple CR, Cardozo L, Cruz F, Hashim H, Michel MC, et al.. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol 2009; 19 (4): 380–394. DOI: 10.1097/mou.0b013e32832ce8a4.
- Rawashdeh YF, Austin P, Siggaard C, Bauer SB, Franco I, Jong TP de, et al.. International children’s continence society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn 2012; 31 (5): 615–620. DOI: 10.1002/nau.22248.
- Franco I, Hoebeke P. Baka-Ostrowska M, et al. Long-term efficacy and safety of solifenacin in pediatric patients aged 6 months to 18 years with neurogenic detrusor overactivity: results from two phase 3 prospective open-label studies. J Pediatr Urol 2019. 6 (2): 80 1–180 8. DOI: 10.1016/j.jpurol.2019.12.012.
- REINBERG Y, CROCKER J, WOLPERT J, VANDERSTEEN D. Therapeutic Efficacy Of Extended Release Oxybutynin Chloride, And Immediate Release And Long Acting Tolterodine Tartrate In Children With Diurnal Urinary Incontinence. J Urol 2003; 169 (317-319): 317–319. DOI: 10.1097/00005392-200301000-00095.
- Baka-Ostrowska M, Bolong DT, Persu C, Tøndel C, Steup A, Lademacher C, et al.. Efficacy and safety of mirabegron in children and adolescents with neurogenic detrusor overactivity: An open-label, phase 3, dose-titration study. Neurourol Urodyn 2021; 40 (6): 1490–1499. DOI: 10.1002/nau.24657.
- Edelstein RA, Bauer SB, Kelly MD. The long-term urologic response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Pediatr Surg 1995; 31 (3): 455–456. DOI: 10.1016/s0022-3468(96)90810-6.
- Karsenty G, Denys P, Amarenco G, De Seze M, Gamé X, Haab F, et al.. Botulinum Toxin A (Botox®) Intradetrusor Injections in Adults with Neurogenic Detrusor Overactivity/Neurogenic Overactive Bladder: A Systematic Literature Review. Eur Urol 2009; 53 (2): 275–287. DOI: 10.1016/j.eururo.2007.10.013.
- Lemelle JL, Guillemin F, Aubert D, Guys JM, Lottmann H, Lortat-Jacob S, et al.. A Multicenter Evaluation of Urinary Incontinence Management and Outcome in Spina Bifida. J Urol 2006; 175 (1): 208???212. DOI: 10.1097/00005392-200601000-00056.
- Metcalfe PD, Rink RC. Bladder augmentation: Complications in the pediatric population. Curr Urol Rep 2007; 8 (2): 152–156. DOI: 10.1007/s11934-007-0065-x.
- Walker RD. The management of the failed bladder neck procedure in patients with spina bifida. BJU Int 2016; 92: 35–37. DOI: 10.1046/j.1464-410x.92.s1.13.x.
- Scales CD, Wiener JS. Evaluating Outcomes of Enterocystoplasty in Patients With Spina Bifida: A Review of the Literature. J Urol 2008; 180 (6): 2323–2329. DOI: 10.1016/j.juro.2008.08.050.
- Kurzrock EA. Pediatric enterocystoplasty: long-term complications and controversies. World J Urol 2009; 27 (1): 69–73. DOI: 10.1007/s00345-008-0335-3.
- Merriman LS, Arlen AM, Kirsch AJ, Leong T, Smith EA. Does augmentation cystoplasty with continent reconstruction at a young age increase the risk of complications or secondary surgeries? J Pediatr Urol 2015; 11 (1): 41.e1–41.e5. DOI: 10.1016/j.jpurol.2014.08.016.
- Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep 2007; 8 (2): 157–162. DOI: 10.1007/s11934-007-0066-9.
- Husmann DA. Malignancy after gastrointestinal augmentation in childhood. Ther Adv Urol 2009; 1 (1): 5–11. DOI: 10.1177/1756287209104163.
- Austin JC. Long-term risks of bladder augmentation in pediatric patients. Curr Opin Urol 2008; 18 (4): 408–412. DOI: 10.1097/mou.0b013e328300587c.
- Arlen AM, Dudley AG, Kieran K. Association of spina bifida with cancer. Transl Androl Urol 2020; 9 (5): 2358–2369. DOI: 10.21037/tau-19-771.
- Kaefer M, Pabby A, Kelly M. Improved Bladder Function After Prophylactic Treatment Of The High Risk Neurogenic Bladder In Newborns With Myelomeningocele. J Urol 1999; 162: 1071. DOI: 10.1097/00005392-199909000-00032.
- Bauer SB, Nijman RJM, Drzewiecki BA, Sillen U, Hoebeke P. International Children’s Continence Society standardization report on urodynamic studies of the lower urinary tract in children. Neurourol Urodyn 2015; 34 (7): 640–647. DOI: 10.1002/nau.22783.
Última actualización: 2023-02-22 15:40