1: 泌尿道的胚胎学
阅读本章大约需要 9 分钟。
引言
尽管泌尿系统与生殖系统在功能上存在差异,这两套系统的胚胎学起源和发育彼此相互整合。泌尿系统由肾脏、输尿管、膀胱和尿道组成。该系统的功能是排泄废物并维持体液与电解质平衡。肾脏还分泌激素,调控血压、促红细胞生成素的产生以及维生素D的合成。生殖系统由性腺、生殖道和外生殖器构成。该系统合成并接受用于生长和发育的激素,产生并运输精子和卵母细胞,并在女性中维持和支持胎儿发育。
对泌尿生殖道胚胎学发育的理解,为理解这些器官的解剖与生理功能以及发育异常结构的病理生理学奠定基础。尽管与各种泌尿生殖系统疾病相关的胚胎学内容在其他章节中已有详细阐述,本章旨在对泌尿生殖道的胚胎学进行概述。
在本概述中,我们使用“男性”指代在46,XY基因型个体中最常见的内生殖器和外生殖器,使用“女性”指代在46,XX基因型个体中最常见的内生殖器和外生殖器。
肾脏和上尿路
肾脏和上尿路的发育经历所提出的前肾、中肾和后肾阶段(图 1) 每个肾的发育最终形成功能性肾实质(由约25万至300万个肾单位1,2),以及集合系统:小肾盏和大肾盏、肾盂和输尿管。
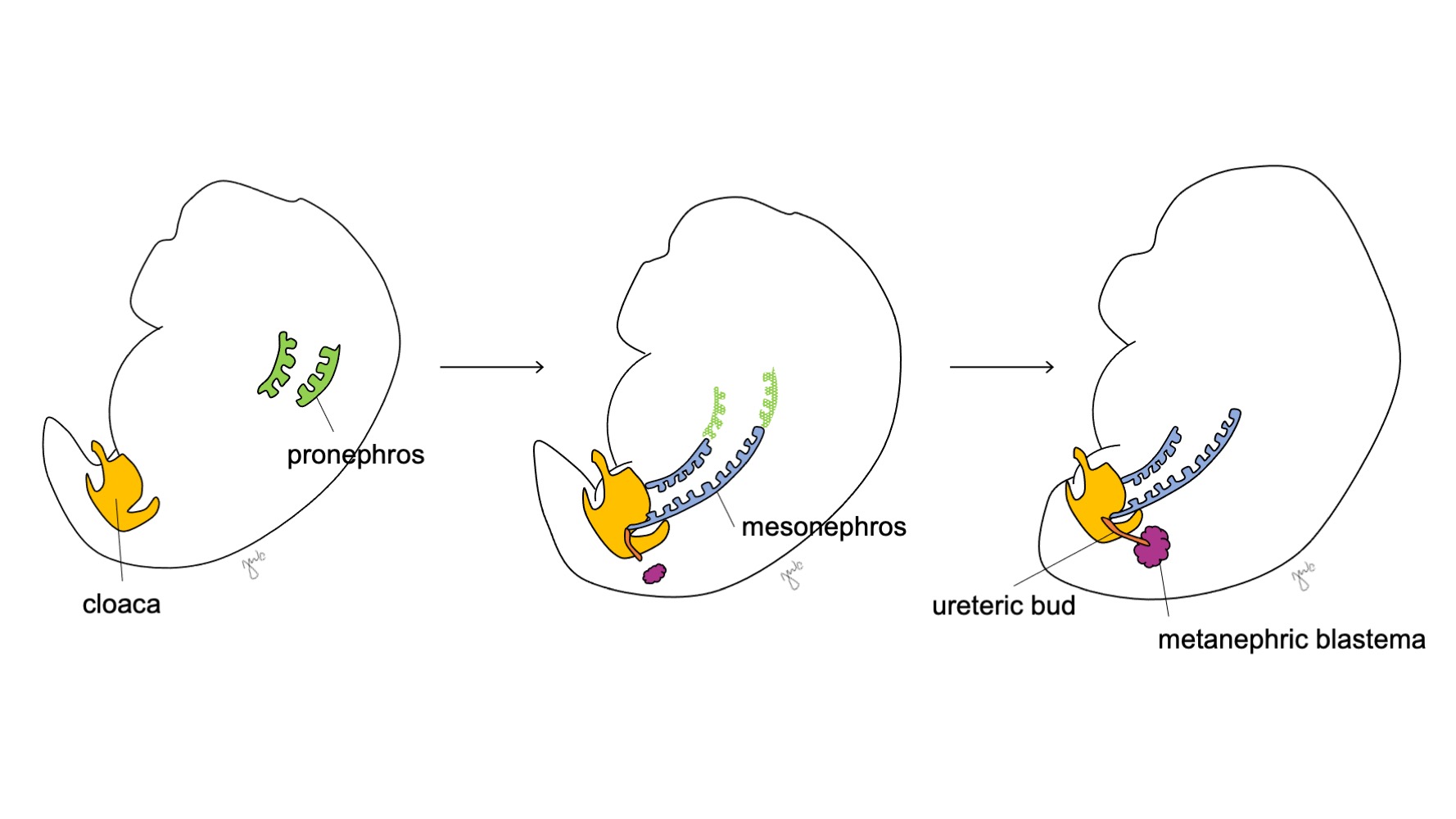
图 1 肾脏及上尿路的发育阶段。 肾脏在妊娠第3周处于前肾阶段(绿色),自第4周开始为中肾阶段(蓝色),妊娠第5周开始为后肾阶段。随着中肾与泄殖腔相连,输尿管芽(橙色)在该连接处旁产生,并与后肾胚芽(栗色)融合。
在妊娠第3周,颈段和上胸段的中间中胚层凝聚并形成前肾。前肾是一种原始且无功能的结构,由组织构成的空心球体组成,似乎在肾的胚胎学发育中仅起短暂作用。前肾管向尾侧延伸,形成中肾管,并于妊娠第4周末退化。基于“个体发生重演系统发生”的观点(即胚胎发育依次经历物种进化的阶段),人类前肾的短暂作用是通过其在多种脊索动物胚胎肾中的功能而被提出的。3 然而,我们对人类前肾知之甚少,而且在对发育3-4周的人类胚胎进行评估时未能检测到前肾结构。3
中肾在妊娠约4周时起源于胸椎水平。3 到第4周末期,中肾向尾侧延伸,与泄殖腔相连。中肾作为原始肾发挥作用,通过小管滤过血液。尽管小管会退化,且随着胚胎生长中肾相对缩小,但中肾(沃尔夫)管在双侧仍可持续存在,并进一步对泌尿道的发育(膀胱三角和输尿管)以及男性生殖道(输精管、附睾、精囊、附睾附件)的发育作出贡献。4
在中肾附着于泄殖腔之后,输尿管芽于妊娠第5周起源于靠近其与泄殖腔连接处的中肾管远端后侧。3 同时,后肾间充质作为由中间中胚层聚集而成的间充质组织团块出现。二者共同形成后肾,这是肾脏及上尿路发育的最终阶段。后肾间充质与输尿管芽相互诱导发育: 后肾间充质包裹并与输尿管芽融合,而输尿管芽穿入后肾间充质并开始分叉。随着输尿管芽延长形成输尿管,它分叉形成肾盂和大肾盏,随后再分支形成小肾盏、肾锥体,并最终形成肾单位的集合小管 (图2) 与此同时,随着输尿管芽延长并分叉,后肾间充质形成肾单位结构 (图3) 血管内皮生长因子-2(VEGF-2)在后肾间充质处启动血管化。5 S形排泄小管获得毛细血管以形成肾小球,每个肾小球外有鲍曼囊包裹。这些排泄小管延长形成近端曲小管、亨利袢和远端曲小管。远端曲小管与集合管(源自输尿管芽)相连,形成肾单位。
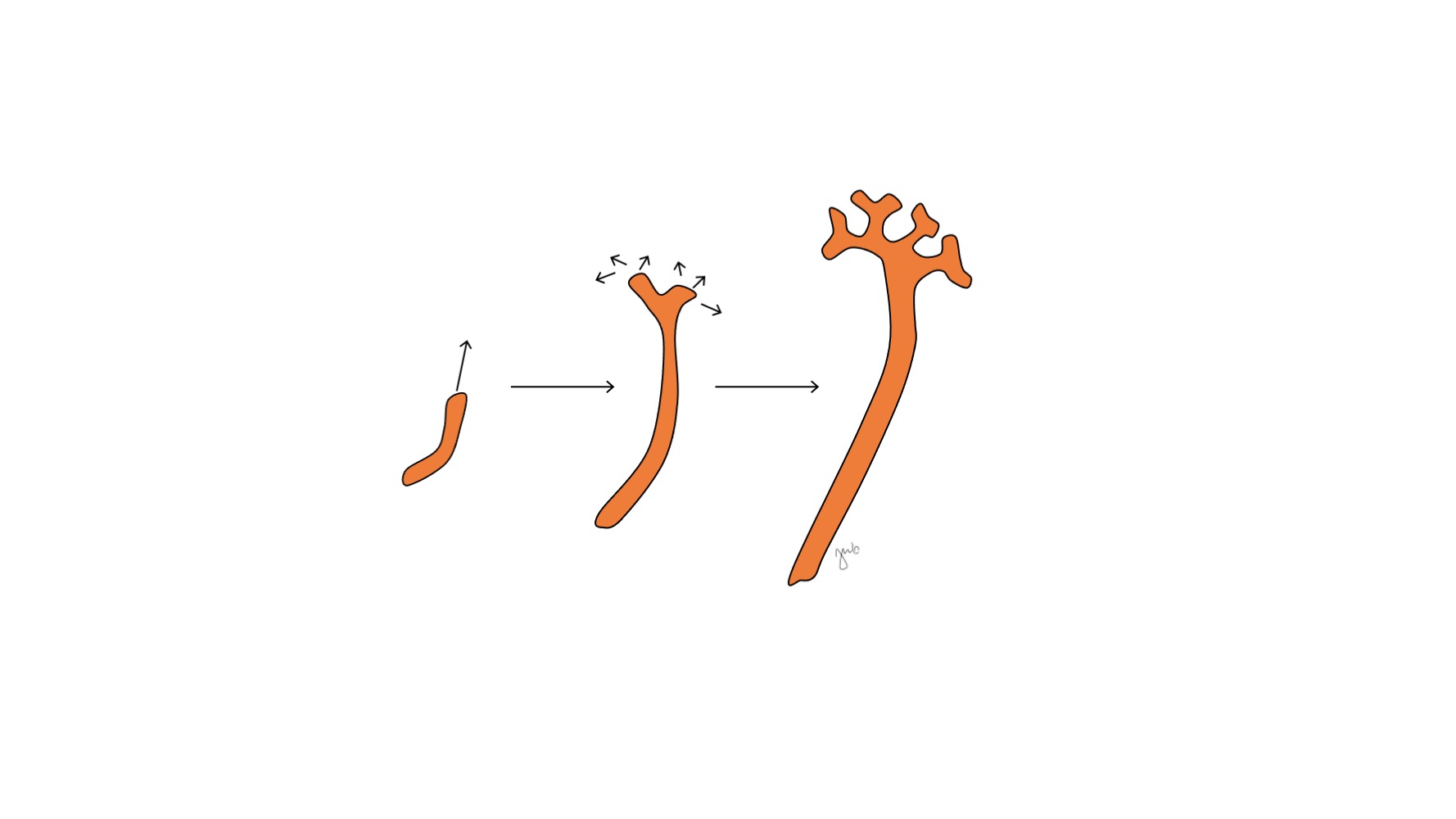
图 2 输尿管芽。 输尿管芽延长并分支,形成肾盂、大肾盏和小肾盏,以及肾单位的集合管。
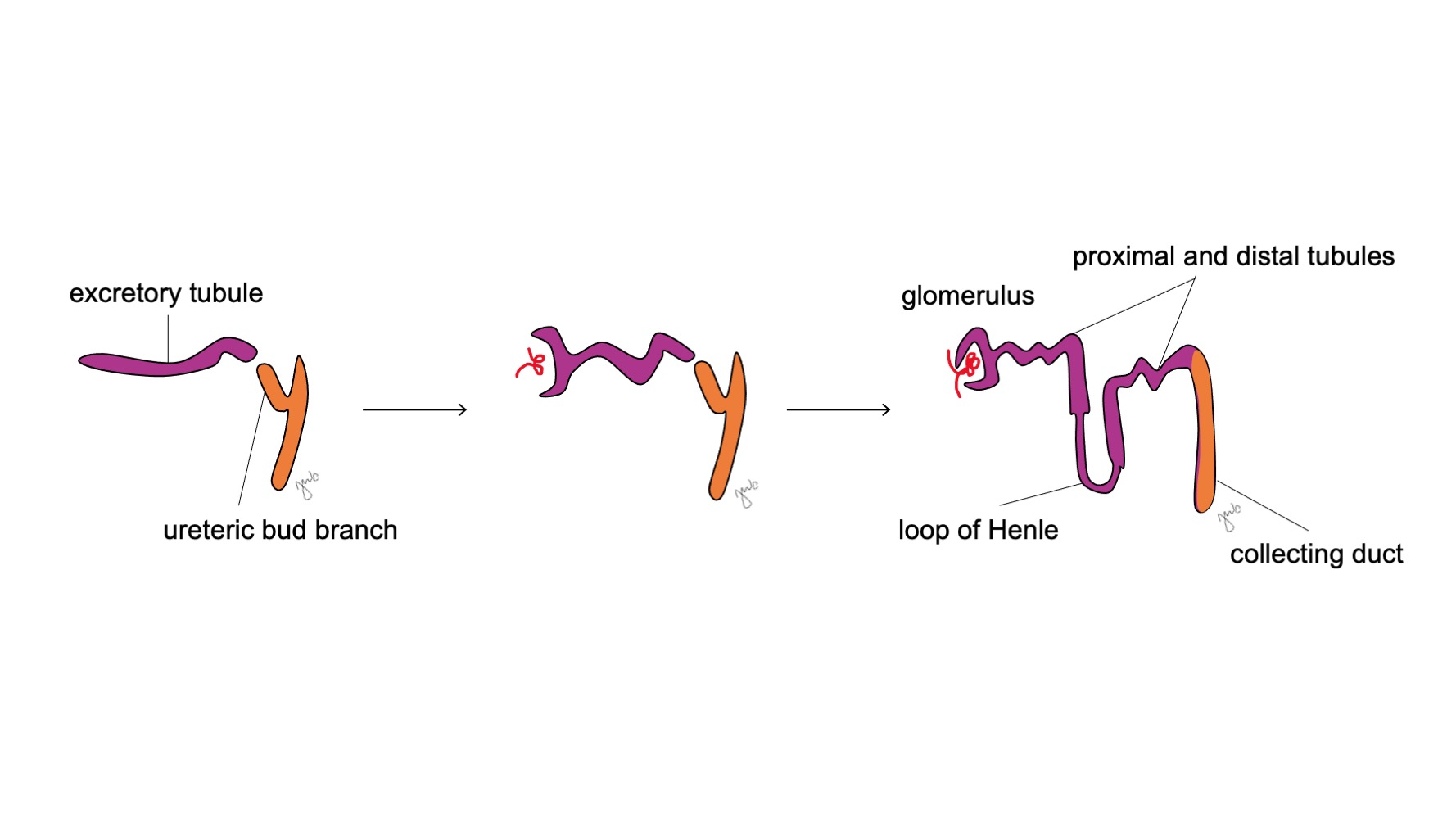
图3 肾单位发育。 排泄性 S 形小管(紫色;来自后肾原基)获得毛细血管形成肾小球,伸长形成近端和远端小管及亨利袢,并与来自输尿管芽的集合管(橙色;源自中肾管)相连,最终形成肾单位。
输尿管芽与后肾间充质通过多种分子因子及其相互作用相互诱导生长,促进后肾的发育。骨形态发生蛋白 BMP-4 在间充质中表达,并具有双重功能:抑制输尿管芽的萌出并促进输尿管延长。6 BMP 拮抗因子 gremlin(grem1)的局部表达使输尿管芽的萌出得以启动。6,7 在输尿管芽开始萌出后,BMP-4 随后促进其延长,并似乎参与促进输尿管周围平滑肌的发育。6,8 来自后肾间充质和输尿管芽的 BMP-7 维持肾单位前体细胞,并提高其对输尿管芽信号的敏感性。6 后肾间充质分泌胶质细胞源性神经营养因子(GDNF),该因子亦可诱导输尿管芽发生分支并延长。9 糖蛋白 WNT11 在分支中的输尿管芽尖端表达,有助于维持 GDNF 的表达。10,11 在后肾发育过程中,WNT11、GDNF 与 RET 酪氨酸激酶受体之间存在相互作用,因为WNT11和RET突变会导致分支缺陷和肾发育不全。11 成纤维生长因子(FGF)配体及其相应受体(FGFR)亦参与后肾间充质的模式化、输尿管芽的诱导、输尿管芽的分支以及肾发生。12 此外,与间充质的直接接触也促进输尿管芽的延长及其分支模式的形成。13
后肾发育成为功能完善且解剖位置正常的肾脏,并伴随早期尿液生成、肾的上升与再血管化,以及肾的旋转。胎儿尿液生成始于妊娠第10-11周,并成为羊水的主要成分。14 起初,肾脏在胚胎的骶部区域彼此相距很近。15,16 随着胚胎纵向生长,各肾在妊娠第6-9周从其最初的盆腔位置上升至位于下胸段与上腰段区域的上部腹膜后(图4) 随着肾脏上升,暂时性血管不断形成并退化,直至其到达上部腹膜后的最终位置,并形成最终的肾动脉和肾静脉。肾脏发生旋转,使肾盂的取向由前向转为内侧。16
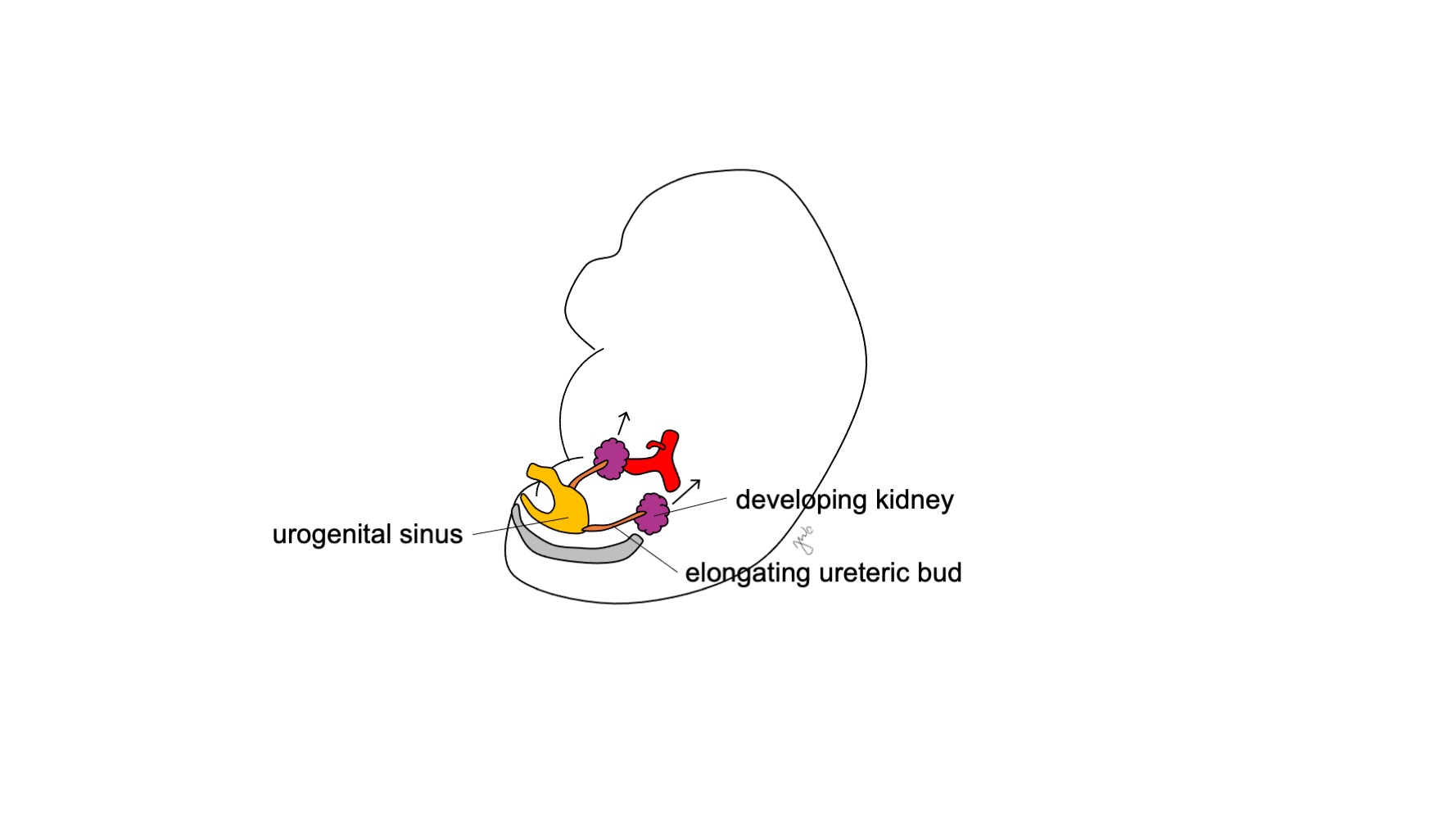
图 4 肾脏上升。 发育中的肾脏从其盆腔位置上升至上部后腹膜间隙。
关键要点
发育中的肾脏和上尿路经历前肾、中肾和后肾阶段。后肾由后肾胚基和输尿管芽组成,它们分别发育为肾实质和集合系统。中肾持续存在,并参与膀胱三角、上尿路集合系统以及男性生殖系统的发育。
下尿路
泄殖腔作为泌尿生殖道与肛门直肠道的共同腔室,它与尿囊(卵黄囊)和后肠相连。它是由外胚层与以内胚层为内衬的结构组合而成,在发育中胚胎的尾侧以单一外口开放。随着中肾附着于泄殖腔且上尿路作为后肾发育,下尿路由泄殖腔发育,形成膀胱和尿道。
大约在妊娠第7周,泌尿直肠隔向尾侧生长,将泄殖腔分隔为前方的泌尿生殖窦和后方的肛直肠管,并最终形成会阴体 (图5)17 随后,泌尿生殖窦再向头侧和尾侧分化,分别形成膀胱和尿道。
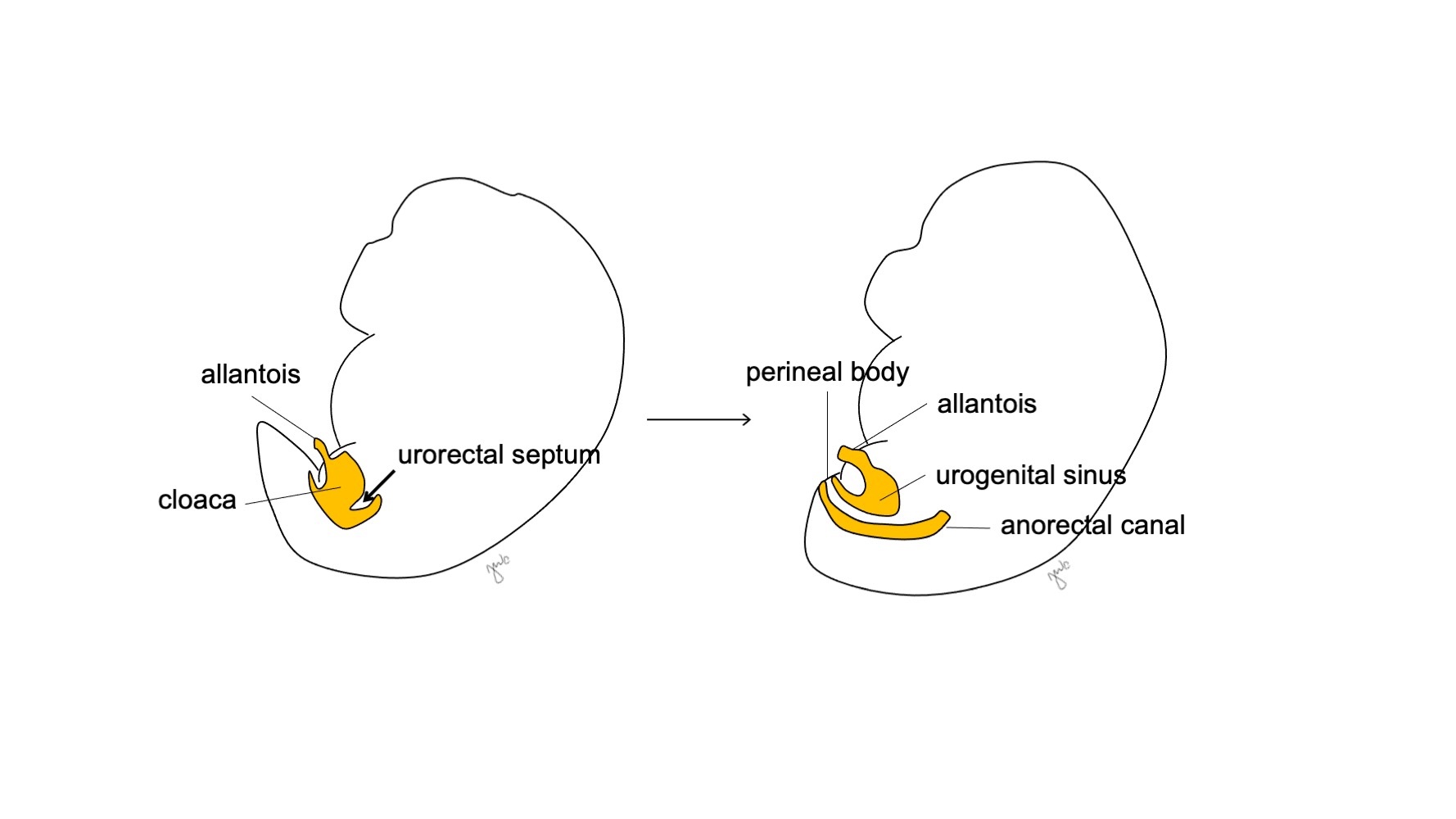
图 5 泄殖腔分隔。 尿直肠隔将泄殖腔分为泌尿生殖窦和肛直肠管。随后尿直肠隔发育为会阴体。
泌尿生殖窦的颅侧部分增大并形成膀胱,起初与尿囊相连续(图 6)同时,原始泄殖腔的内胚层形成膀胱的尿路上皮内衬。18 膀胱平滑肌的分化依赖于间充质-上皮相互作用。19 先前连于泄殖腔的中肾管仍与发育中的膀胱相连;其后退化,并在尾侧被并入膀胱三角和输尿管口。维生素A诱导的中肾管凋亡、膀胱与输尿管平滑肌之间的相互作用以及内胚层来源,是被提出的促成膀胱三角区发育的其他机制。20,21,22
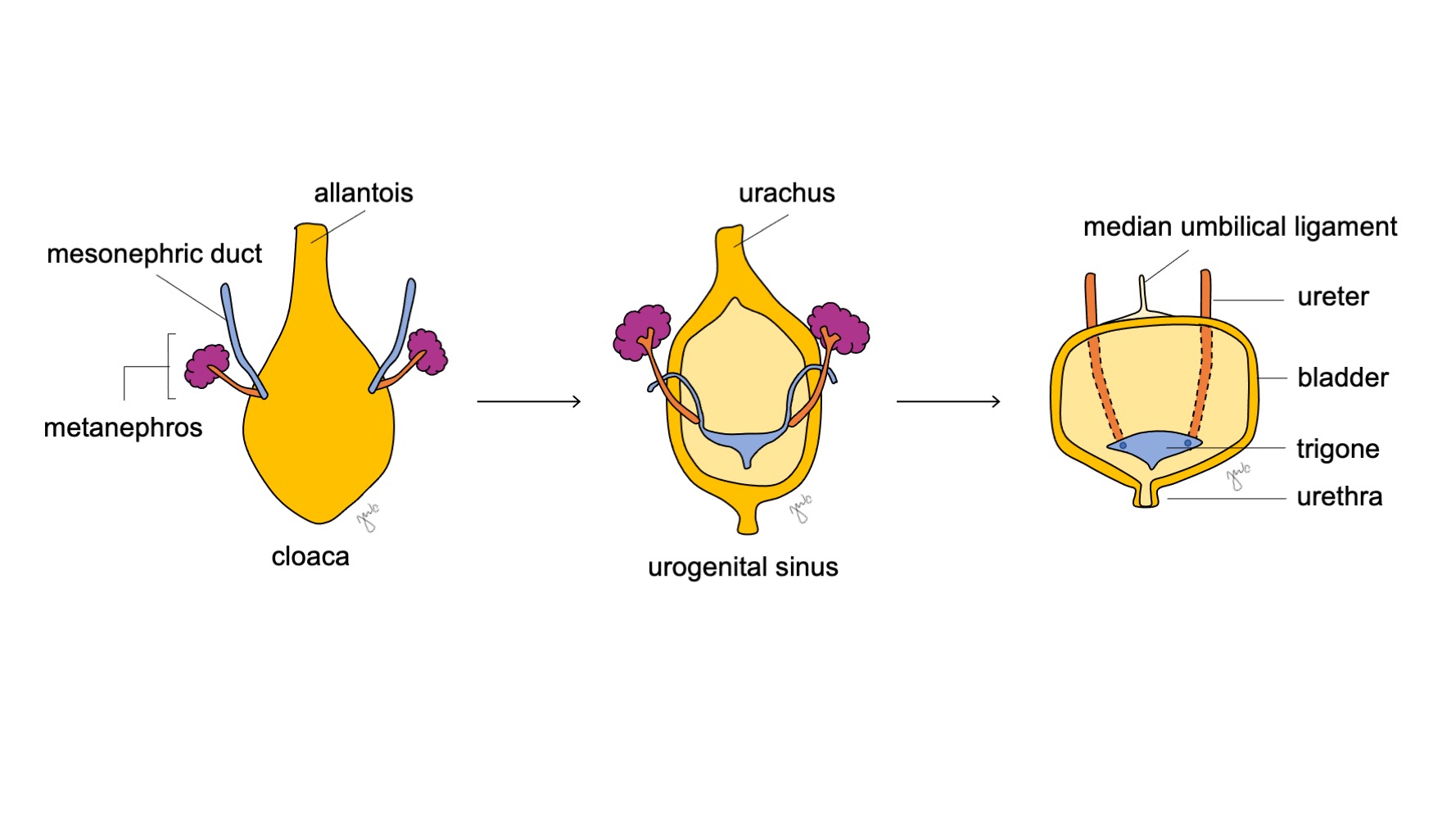
图 6 膀胱发育。 泌尿生殖窦的头侧部分增大并发育为膀胱。中肾管(蓝色)退行并并入膀胱三角区和输尿管口。与此同时,尿囊退化,形成尿膜管,随后进一步纤维化为正中脐韧带。
中肾管的其余部分随后发育为男性生殖道(详见性腺与生殖道部分)。随着胚胎继续生长,中肾管的衍生结构彼此进一步分离:随着男性生殖道向尾侧并向内侧移动,输尿管口向颅外侧移动。相对于中肾管位置的输尿管芽移位会影响输尿管口的最终位置以及相应肾脏的发育。23,24 较轻的移位可导致输尿管口位于颅外侧或尾内侧位置,而严重移位可导致异位输尿管开口于膀胱外和/或肾发育不良。23,24
随着膀胱发育,尿囊逐渐退化,形成连接膀胱顶与脐部的脐尿管。出生后,脐尿管残余进一步纤维化,成为正中脐韧带。25
泌尿生殖窦的尾侧下部在男性形成前列腺部和膜部尿道,在女性形成尿道和阴道前庭。25 与膀胱类似,尿道的移行上皮来源于内胚层。18 在男性,泌尿生殖窦向生殖结节延伸以形成前列腺部和膜部尿道,而其相关的泌尿生殖褶形成阴茎尿道。18,26 妊娠约9-10周时,来自前列腺部尿道的进一步分支与外芽形成前列腺腺体。27 前列腺基质源自间充质,并在雄激素以及多种因子的旁分泌信号影响下发育,这些因子包括 FGF、sonic hedgehog (SHH)、转录因子 Nkx3.1、同源盒 (HOX) 基因、叉头框 (FOX) 转录因子、SRY-box 转录因子 (SOX-9)、BMP 和 WNT。27,28,29,30,31 虽然睾酮可促进前列腺发育,但二氢睾酮 (DHT) 对前列腺的作用更为强大。27 前列腺间充质形成基质组织以及围绕前列腺腺体和导管的平滑肌。男性的球尿道腺 (库珀氏腺) 由尿道出芽形成。阴茎尿道是在尿道板发生管腔化时形成的18,26 (在外生殖器部分讨论)。目前关于尿道龟头部的发育仍存在争议,并且基于较新的研究,其可能由管腔化形成,随后内胚层分化为鳞状上皮。18,32,33
在女性中,泌尿生殖窦形成整个尿道和阴道的远端。与男性的前列腺腺体同源,尿道的头侧部分形成尿道旁腺(斯基恩腺)。与男性的球尿道腺(库珀腺)同源,前庭大腺(巴氏腺)由尿道出芽形成。
要点
泄殖腔最初为一个共同的腔室,由尿直肠隔分隔,形成泌尿生殖窦和肛直肠管。泌尿生殖窦随后向颅侧扩大形成膀胱,并在两性中均参与尿道的形成。与此同时,尿囊退化形成脐尿管,后者随后成为正中脐韧带。
性腺与生殖道
男性和女性的性腺及内生殖道最初均未分化。在遗传和激素因素的影响下,发育将分化为睾丸及其相关的中肾(Wolffian)结构,或卵巢及其相关的副中肾(Müllerian)结构。
在妊娠第5周,生殖上皮和间充质的增殖与凝聚在中肾旁形成生殖嵴。始基生殖细胞与称为性索的上皮簇一起被并入。性索向局部间充质组织内陷,并促使始基生殖细胞在6周时经卵黄囊腔迁移至生殖嵴。34 随着生殖细胞与性索在生殖嵴处聚集,性腺的皮质和髓质区域开始发育。35,36 WNT4 和 SOX-9 因子在生殖嵴中表达,并且在人类性别决定过程中其表达有所不同。37 性腺的形成还受到包括 Wilms 瘤 1(WT1)、LIM 同源盒 9(LHX9)和类固醇生成因子 1(SF1)在内的转录因子的调控。38
未分化性腺具有双向分化潜能(图 7),原始生殖细胞在妊娠第七周之前仍可分化为男性的精原细胞或女性的卵原细胞。38 中肾管(沃尔夫管)和副中肾管(苗勒管)此时也具有双向分化潜能。
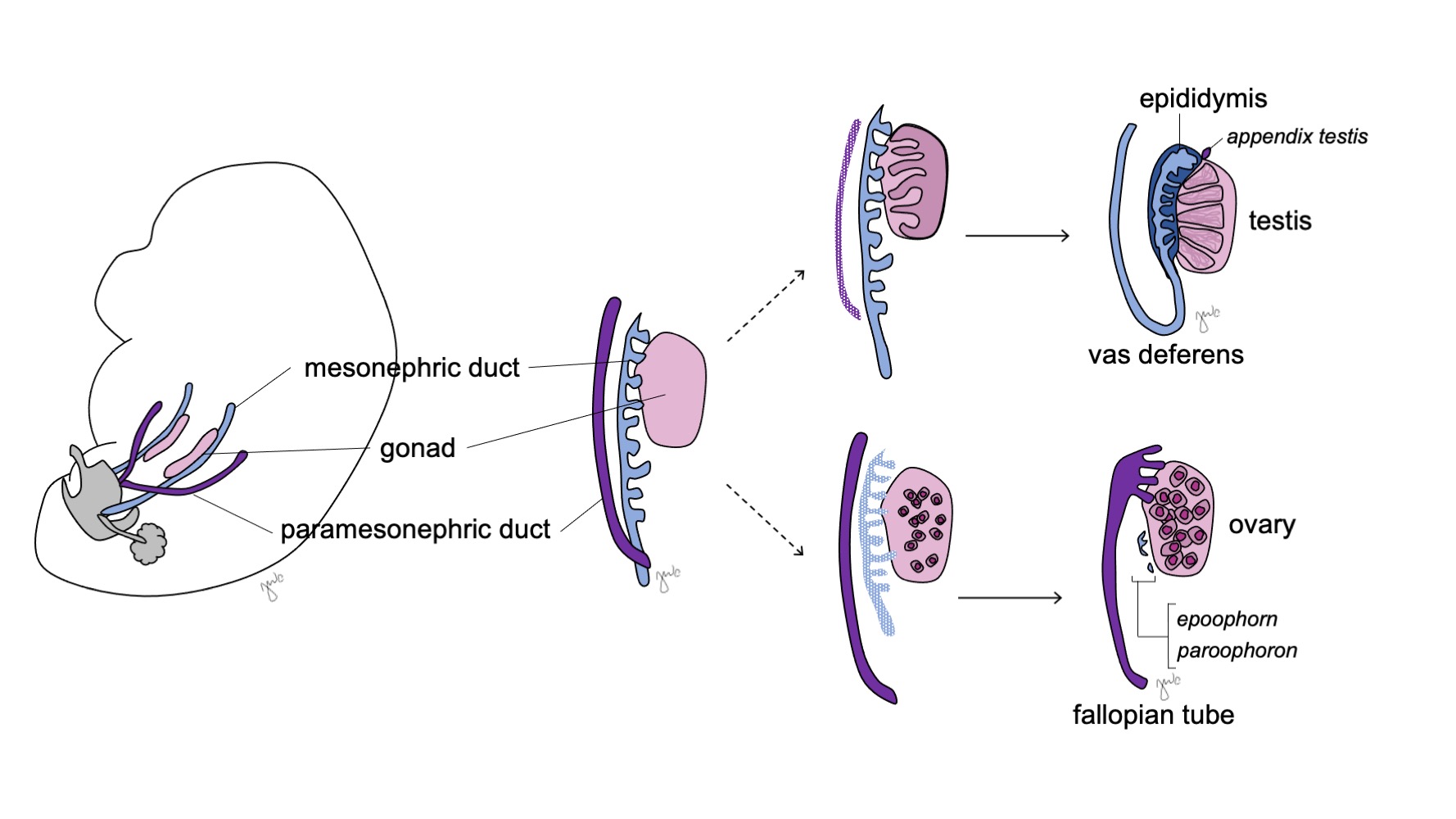
图 7 性腺分化。 性腺(粉色)通常分化为伴随相应的中肾管结构(蓝色)的睾丸,或伴随相应的旁中肾管结构(紫色)的卵巢。
男性性腺与生殖道
在男性中,未分化的性腺发育为睾丸,而中肾管(沃尔夫管)发育为附睾、附睾小体、输精管、精囊和射精管。
SRY(s性别决定 r区域,位于 Y-染色体上)基因(亦称睾丸决定因子 [TDF])位于Y染色体短臂Yp11.3,并启动男性性分化。39,40 相应的SRY蛋白以及来源于SOX-9、DAX-1、WT1和SF1基因的转录因子会影响性腺分化,.% cite mcelreavey1995a vilain1998a –file 01-01 %} 已发现SRY上调SOX-9以激活SF1的表达,并促进塞尔托利细胞和莱迪希细胞的分化。37,41,42 原始性腺性索组织为睾丸网和生精索。塞尔托利细胞和莱迪希细胞在生精索之间发育,并开始产生激素以促进男性分化(见下文)。睾丸白膜包裹这些索以形成睾丸。生精索最终形成腔,至青春期成为曲细精管。
Sertoli细胞和Leydig细胞随后开始产生睾丸激素,引发一系列男性化过程。Sertoli细胞在SOX-9、SF1、WT1和DAX1以及促卵泡激素(FSH)的影响下分泌米勒管抑制因子(MIF)或抗米勒管激素(AMH)。43 随后,抗米勒管激素导致副中肾管(米勒管)退化,其残余为睾丸小体和前列腺小囊。40,43,44 SOX-9与SF1相互作用以增强AMH的效应,促使米勒管退化。Leydig细胞同时对黄体生成素(LH)和结构相似的人绒毛膜促性腺激素(hCG)作出反应,于第8-9周启动睾酮的产生。睾酮诱导中肾管(图8)分化为附睾、附睾小体、输精管、射精管和精囊,并通过转化为二氢睾酮(DHT)使外生殖器男性化。
在胚胎发育过程中,睾丸下降始于第10周,并取决于腹腔内压力、激素影响以及来自睾丸引带的张力。45 当睾丸进入深(内)腹股沟环并形成腹股沟管时,腹膜的一层形成鞘膜。睾丸在第20-28周之间通过腹股沟管,并经浅(外)腹股沟环进入阴囊。前腹壁的腹外斜肌、腹内斜肌和腹横筋膜分别形成覆盖睾丸的相应阴囊层,依次为外精索筋膜、提睾肌筋膜与肌肉以及内精索筋膜。45
女性性腺与生殖道
在女性中,未分化的性腺发育为卵巢,副中肾管(苗勒氏管)发育为输卵管、子宫、宫颈和近端阴道。
在46,XX基因型者或为46,XY基因型但SRY基因缺失者中,卵巢在缺乏SRY基因的情况下发育。46 卵巢皮质增生并形成向性腺髓质延伸的原发性性索。4个月时,这些性索分隔并形成由卵原细胞和卵泡细胞构成的原始卵泡。卵原细胞进行有丝分裂,而初级卵母细胞在出生时停滞于减数分裂Ⅰ前期,作为未来生殖所需的卵泡储备。42 与睾丸下降相比,卵巢在更短的时间内下降进入盆腔。45
女性性腺发育先前被认为是在缺乏SRY基因影响时发生的“默认”机制。然而,卵巢发育似乎并非一个简单的被动过程。它在WNT4和DAX-1的遗传和分子层面的作用下得到主动促进。37,40,41,42 这两种基因均具有抗睾丸作用,而WNT4被认为是“卵巢决定基因”。37,40 WNT4通过上调DAX-1的表达来抑制SF1的转录活性,抑制SOX-9的功能,并最终促进卵巢发育。41,42
在性分化之前,副中肾(苗勒)管起源于位于中肾(沃尔夫)管外侧的上皮内陷。47 胚胎发育早期缺乏 AMH,使副中肾(苗勒)管得以发育为输卵管、子宫、宫颈和阴道近端(图 8)。42,44,48 这些管的外侧部分形成输卵管。副中肾(苗勒)管的内侧部分在中线融合形成子宫,联合后的管道远端与泌尿生殖窦接触,使阴道的近端与远端相接(图 9)。44,47,49 周围的间充质分化为子宫的子宫内膜和子宫肌层。48 类似于在男性中副中肾(苗勒)管退化为睾丸阑尾,女性的中肾管退化为邻近阴道的卵巢上体、卵巢旁体和 Gartner 管。44
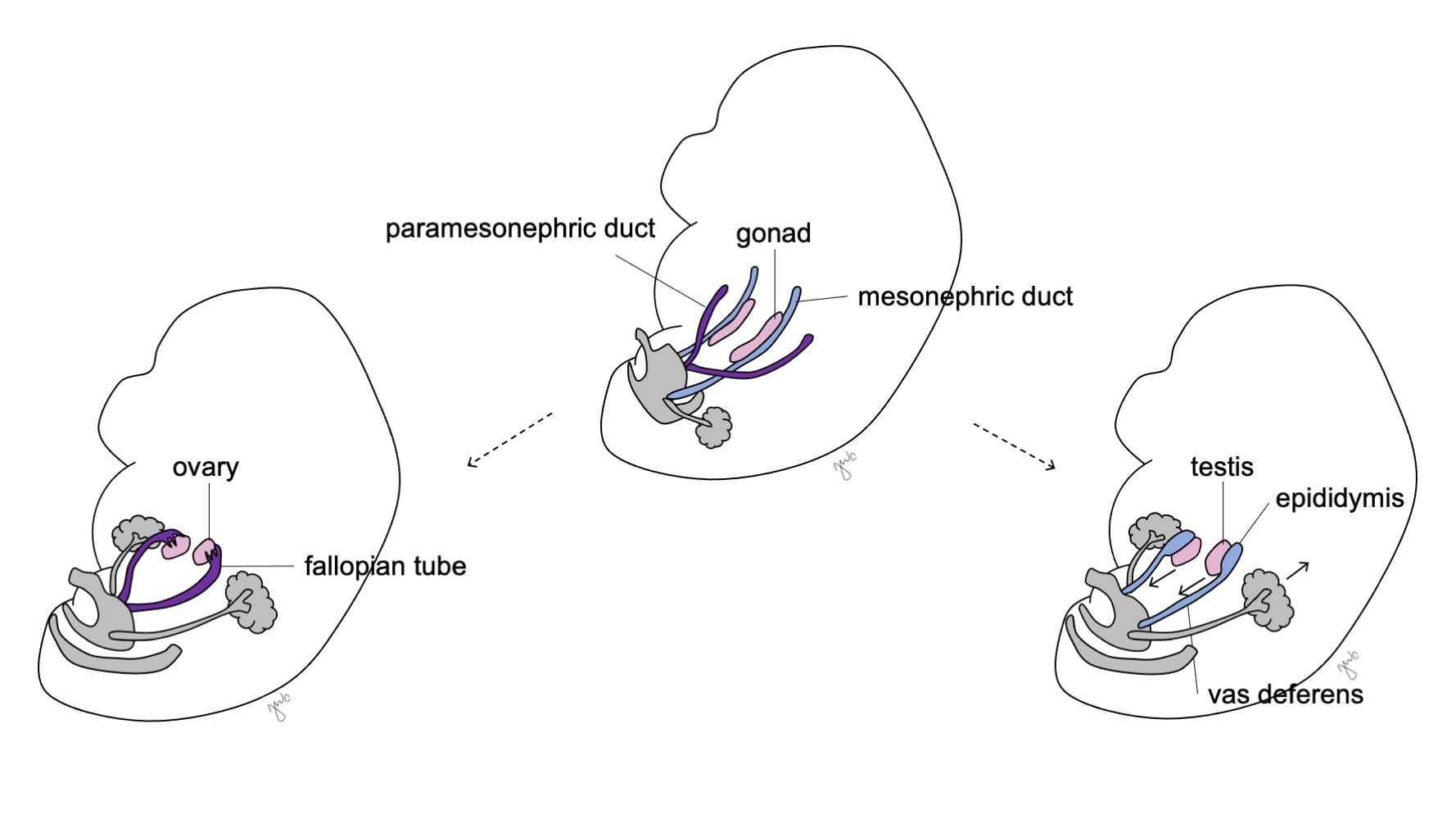
图 8 中肾管与副中肾管的分化。 在男性中,中肾管(蓝色)分化为附睾、输精管、射精管和精囊。在女性中,副中肾管(紫色)分化为输卵管、子宫、宫颈和阴道近端。
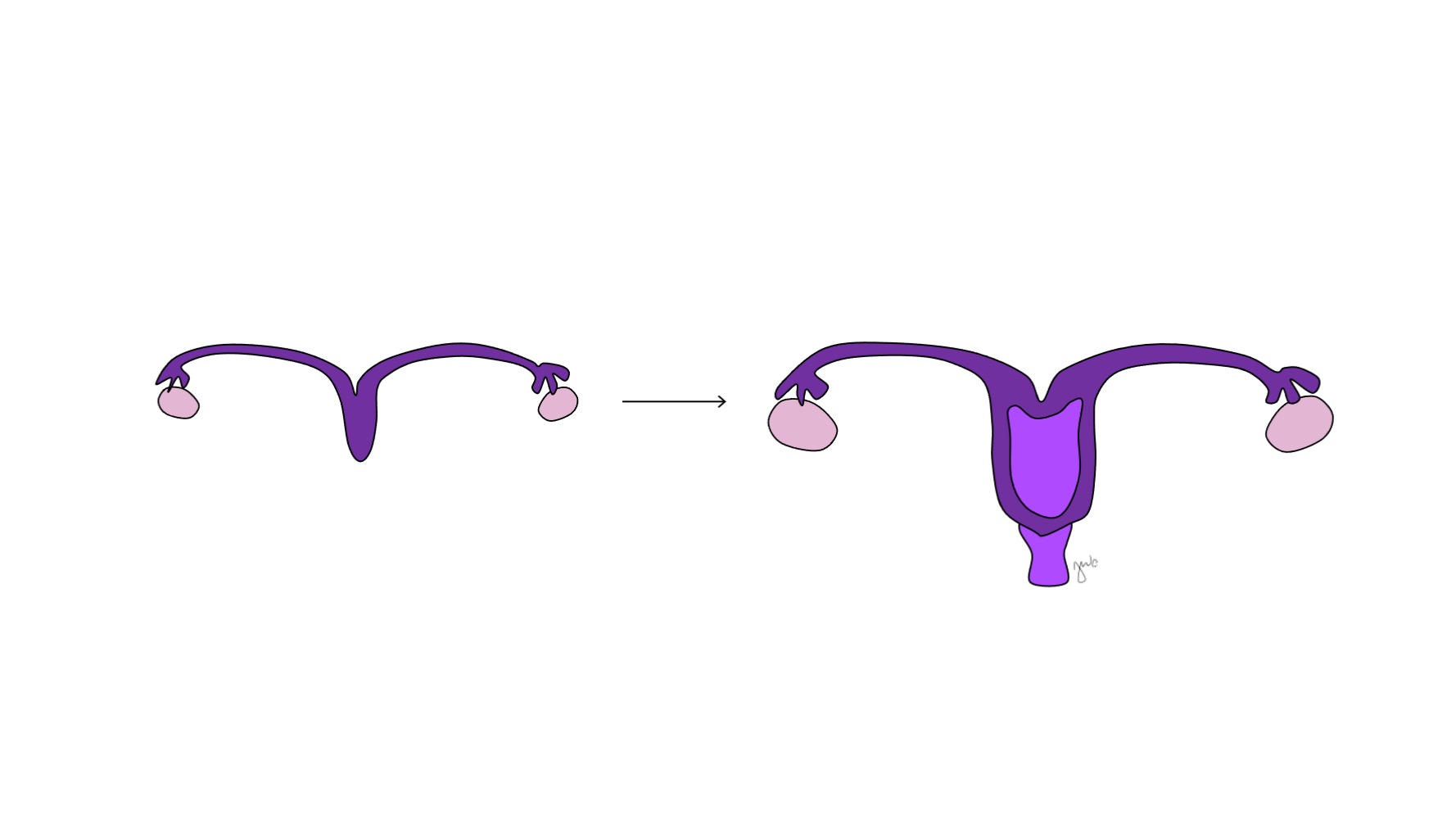
图 9 副中肾管融合。 副中肾管的内侧部分在中线融合,形成子宫。该融合结构的远端形成宫颈和近端阴道,随后向尾侧与泌尿生殖窦相连。
类似于性腺发育,女性生殖道的发育也不是一种被动的”默认”机制,因为WNT4信号对于苗勒氏管的发育和分化以及抑制雄性分化至关重要。42,48 同源盒基因有助于旁中肾(苗勒氏)结构的前后轴排列。48 与睾丸的支持细胞类似,卵巢颗粒细胞最终也会分泌AMH,但这一过程发生在胎儿发育的更晚期,即在旁中肾(苗勒氏)结构已经形成之后。43
要点
性腺和导管的分化概述见表 1。未分化的性腺和原始生殖细胞可发育为含有精原细胞的睾丸或含有卵原细胞的卵巢。随后,这些性腺分泌的激素影响中肾管(沃尔夫管)发育为附睾、附睾小体、输精管、射精管和精囊,或影响副中肾管(苗勒管)发育为输卵管、子宫、宫颈和阴道近端。
外生殖器
外生殖器在妊娠第七周之前均为两性潜能状态。随着泄殖腔分离为泌尿生殖窦和肛直肠管(此前在下尿路部分已讨论),其两侧形成褶。待泌尿直肠隔完成对泄殖腔的分隔后,侧方的褶成为泌尿生殖褶,而褶的前部融合形成生殖结节。位于生殖结节两侧的隆起形成阴唇阴囊隆起。生殖结节、泌尿生殖褶和阴唇阴囊隆起(图 10)在与其同时发育的性腺所分泌的激素影响下发生分化。
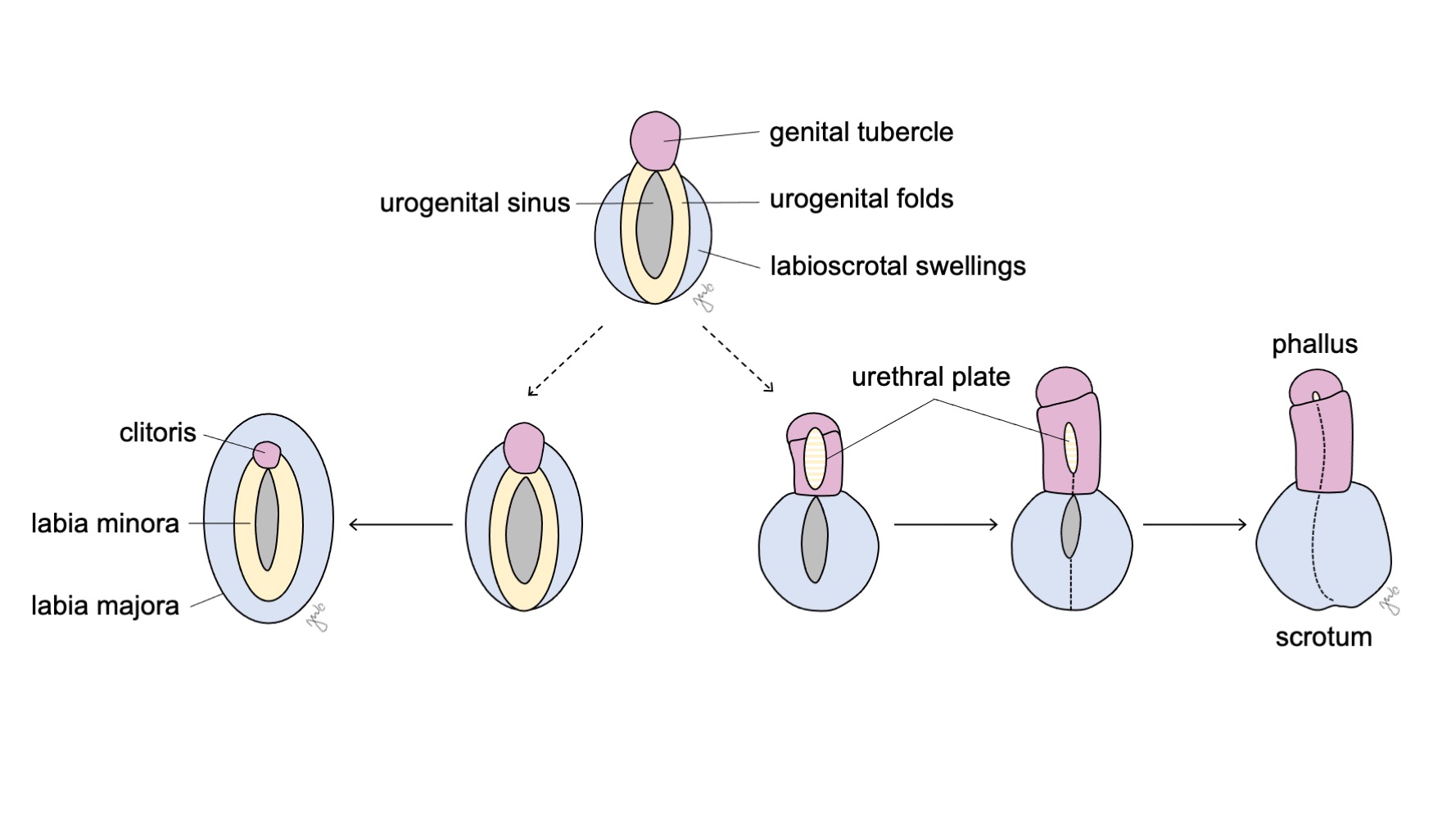
图 10 外生殖器的分化。 生殖结节(粉色)、泌尿生殖褶(黄色)和阴唇阴囊隆起(蓝色)分别在女性中分化为阴蒂、小阴唇和大阴唇,在男性中分化为阴茎、尿道板和阴囊。
男性外生殖器
来自睾酮和二氢睾酮 (DHT) 的雄激素影响导致泌尿生殖窦和外生殖器男性化,形成阴茎和阴囊 (图 10).34,44
雄激素暴露使生殖结节在妊娠8-12周发生伸长,此后自妊娠14周起呈线性生长,自妊娠18周起超声即可检出阴茎结构。34,50,51 SHH 在生殖结节和尿道板上皮中表达,可能促进雄激素对阴茎形成及其他形式的生殖器男性化的作用。34,52 阴茎间充质凝聚形成阴茎海绵体和尿道海绵体。53 在胎儿中已观察到阴茎腹侧弯曲,可持续至妊娠第20th周,随后在余下的妊娠期逐渐消退。54
泌尿生殖襞在中线融合,形成尿道板,作为阴茎尿道发育的一部分。尿道板中线的沟逐渐加深,板的上皮缘融合形成管状结构。33,55 由此融合形成的上皮缝随后被重塑,并被间充质细胞取代。55 阴茎尿道的近端在尾侧与源自泌尿生殖窦的前列腺部尿道融合的同时,管化过程向远侧和腹侧推进。如前所述,龟头部尿道的发育仍存在争议。尽管过去将该段尿道归因于来自龟头尖端的外胚层内陷,但较新的研究提出,龟头部尿道通过管化形成,随后内胚层分化为鳞状上皮。18,32,33 随着生殖突延长且尿道板发生管化,阴唇阴囊隆起在中线的正中缝处融合,形成阴囊。56
女性外生殖器
尽管缺乏雄激素可避免男性化,女性外生殖器的发育并非 “默认” 过程,因为两条X染色体、卵巢发育以及雌激素均对该过程有贡献。遗传、性腺和激素影响对发育的重要性,可由缺失一条X染色体的特纳综合征患者所见的外生殖器发育不全,或关于X染色体易位的报告加以证明。57,58
在雌激素的作用以及雄激素缺乏的共同影响下,泌尿生殖窦和外生殖器发育为阴蒂、大阴唇和小阴唇,以及阴道下段(图 10) 在缺乏雄激素的情况下,生殖结节退化并形成阴蒂。泌尿生殖窦(最初在泄殖腔分隔后形成)发育形成女性整个尿道和阴道远端。阴道远端向颅侧与副中肾管融合。中央细胞退化形成阴道腔,后壁内陷形成处女膜。尿生殖褶成为小阴唇,其后方轻度融合形成阴唇后连合。女性的阴唇阴囊隆起保持不融合,形成大阴唇。
表 1 性分化与发育。
| 男性 | 女性 | |
|---|---|---|
| 性腺 | ||
| 原始生殖细胞 | 精原细胞 | 卵原细胞 |
| 性索 | 睾丸索 | 原始卵泡 |
| 中肾(沃尔夫)管 | 附睾, 附睾附属体, 输精管, 精囊, 射精管 | 加特纳管, 卵巢上体, 卵巢旁体 |
| 副中肾(苗勒)管 | 睾丸附属体, 前列腺小囊 | 输卵管, 子宫, 宫颈, 阴道近端 |
| 尿生殖窦 | 膀胱, 尿道近端, 可能包括龟头部远端尿道 | 膀胱, 尿道, 阴道远端 |
| 外生殖器 | ||
| 生殖结节 | 阴茎 | 阴蒂 |
| 尿生殖襞 | 尿道板 | 小阴唇 |
| 阴唇阴囊隆起 | 阴囊 | 大阴唇 |
要点
外生殖器的分化亦在表 1中概述。生殖结节在男性发育为阴茎,在女性发育为阴蒂。尿生殖皱襞在男性形成尿道板,在女性形成小阴唇。阴唇阴囊隆起在男性发育为阴囊,在女性发育为大阴唇。作为下尿路发育的一部分,尿生殖窦在男性形成近端尿道,其尿生殖皱襞形成远端尿道的尿道板。相较之下,女性的尿生殖窦形成整个尿道以及阴道远段。
结论
泌尿道和生殖道的发育通过其胚胎学起源及相互作用而相互整合。发育中的肾脏经历原肾、中肾和后肾阶段。中肾与泄殖腔之间的相互作用产生输尿管芽和后肾间充质,最终发育为肾脏和上尿路。泄殖腔同时发生分隔,前部泌尿生殖窦发育为膀胱,并参与男女尿道的形成。生殖腺、中肾管(沃尔夫管)和副中肾管(苗勒管)、以及外生殖器具有沿男性与女性性分化谱发育的潜能:遗传与激素因素分别影响性腺功能和发育中胚胎的表型表现。涉及这些过程的基因、分子相互作用或发育中组织的各种改变,均可影响相应结构的长期解剖形态与功能。
参考文献
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol 2011; 26 (9): 1529–1533. DOI: 10.1007/s00467-011-1843-8.
- Tryggvason K, Kouvalainen K. Number of Nephrons in Normal Human Kidneys and Kidneys of Patients with the Congenital Nephrotic Syndrome. Nephron 1975; 15 (1): 62–68. DOI: 10.1159/000180493.
- Bakker BS de, Hoff MJB van den, Vize PD, Oostra RJ. The Pronephros; a Fresh Perspective. Integr Comp Biol 2019; 59 (1): 29–47. DOI: 10.1093/icb/icz001.
- Ludwig KS, Landmann L. Early development of the human mesonephros. Anat Embryol (Berl) 2005; 209 (6): 439–447. DOI: 10.1007/s00429-005-0460-3.
- Nagata M. Glomerulogenesis and the role of endothelium. Curr Opin Nephrol Hypertens 2018; 27 (3): 159–164. DOI: 10.1097/mnh.0000000000000402.
- Nishinakamura R, Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatr Nephrol 2014; 29 (4): 681–686. DOI: 10.1007/s00467-013-2671-9.
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 2004; 131 (14): 3401–3410. DOI: 10.1242/dev.01251.
- Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D. Antagonism of BMP4 Signaling Disrupts Smooth Muscle Investment of the Ureter and Ureteropelvic Junction. J Urol 2009; 181 (1): 401–407. DOI: 10.1016/j.juro.2008.08.117.
- Sajithlal G, Zou D, Silvius D, Xu P-X. Eya1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol 2005; 284 (2): 323–336. DOI: 10.1016/j.ydbio.2005.05.029.
- Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev 2004; 14 (5): 550–557. DOI: 10.1016/j.gde.2004.07.009.
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11andRet/Gdnfpathways cooperate in regulating ureteric branching during metanephric kidney development. Development 2003; 130 (14): 3175–3185. DOI: 10.1242/dev.00520.
- Walker KA, Sims-Lucas S, Bates CM. Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr Nephrol 2016; 31 (6): 885–895. DOI: 10.1007/s00467-015-3151-1.
- Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymal–epithelial contact in the developing kidney. Proc Natl Acad Sci U S A 1999; 96 (13): 7330–7335. DOI: 10.1073/pnas.96.13.7330.
- Underwood MA, Gilbert WM, Sherman MP. Amniotic Fluid: Not Just Fetal Urine Anymore. J Perinatol 2005; 25 (5): 341–348. DOI: 10.1038/sj.jp.7211290.
- Taghavi K, Kirkpatrick J, Mirjalili SA. The horseshoe kidney: Surgical anatomy and embryology. J Pediatr Urol 2016; 12 (5): 275–280. DOI: 10.1016/j.jpurol.2016.04.033.
- Friedland GW, De Vries P. Renal ectopia and fusion. Urology 1975; 5 (5): 698–706. DOI: 10.1016/0090-4295(75)90137-5.
- Kruepunga N, Hikspoors JP, Mekonen HK, Mommen GM, Meemon K, Weerachatyanukul W, et al.. Faculty Opinions recommendation of The development of the cloaca in the human embryo. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2018; 33 (6): 24–39. DOI: 10.3410/f.734193252.793568763.
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 2008; 318 (1): 143–152. DOI: 10.1016/j.ydbio.2008.03.017.
- Baskin LS, Hayward SW, Young P, Cunha GR. Role of Mesenchymal-Epithelial Interactions in Normal Bladder Development. J Urol 1996; 56 (5): 1820–1827. DOI: 10.1097/00005392-199611000-00101.
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al.. Faculty Opinions recommendation of Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2005; 7 (10): 082–089. DOI: 10.3410/f.1029077.343528.
- Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, et al.. The development of the bladder trigone, the center of the anti-reflux mechanism. Development 2007; 134 (20): 3763–3769. DOI: 10.1242/dev.011270.
- Tanaka ST, Ishii K, Demarco RT, Pope JC, Brock JW, Hayward SW. Endodermal Origin of Bladder Trigone Inferred From Mesenchymal-Epithelial Interaction. J Urol 2010; 183 (1): 386–391. DOI: 10.1016/j.juro.2009.08.107.
- Mackie GG, Stephens FD. Duplex Kidneys: A Correlation of Renal Dysplasia with Position of the Ureteral Orifice. J Urol 1975; 114 (2): 274–280. DOI: 10.1016/s0022-5347(17)67007-1.
- Wen JG, Frøkiaer J, Zhao JB, Ringgaard S, Jørgensen TM, Djurhuus JC. Severe partial ureteric obstruction in newborn rats can produce renal dysplasia. BJU Int 1981; 89 (7): 740–745. DOI: 10.1046/j.1464-410x.2002.02747.x.
- Parada Villavicencio C, Adam SZ, Nikolaidis P, Yaghmai V, Miller FH. Imaging of the Urachus: Anomalies, Complications, and Mimics. Radiographics 2016; 36 (7): 2049–2063. DOI: 10.1148/rg.2016160062.
- Krishnan A, Souza A de, Konijeti R, Baskin LS. The Anatomy and Embryology of Posterior Urethral Valves. J Urol 2006; 175 (4): 1214–1220. DOI: 10.1016/s0022-5347(05)00642-7.
- Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, et al.. A comparison of prostatic development in xenografts of human fetal prostate and human female fetal proximal urethra grown in dihydrotestosterone-treated hosts. Differentiation 2018; 115 (24-45): 37–52. DOI: 10.1016/j.diff.2020.06.001.
- Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction 2001; 21 (2): 187–195. DOI: 10.1530/rep.0.1210187.
- LASNITZKI ILSE, MIZUNO TAKEO. Prostatic Induction: Interaction Of Epithelium And Mesenchyme From Normal Wild-type Mice And Androgen-insensitive Mice With Testicular Feminization. J Endocrinol 1980; 85 (3): 423–np. DOI: 10.1677/joe.0.0850423.
- Meeks JJ, Schaeffer EM. Genetic Regulation of Prostate Development. J Androl 2011; 32 (3): 210–217. DOI: 10.2164/jandrol.110.011577.
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol 2003; 253 (2): 165–174. DOI: 10.1016/s0012-1606(02)00031-3.
- Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: Theory of endodermal differentiation. Differentiation 1999; 64 (2): 115–122. DOI: 10.1046/j.1432-0436.1999.6420115.x.
- Hadidi AT, Roessler J, Coerdt W. Development of the human male urethra: A histochemical study on human embryos. J Pediatr Surg 2014; 49 (7): 1146–1152. DOI: 10.1016/j.jpedsurg.2014.01.009.
- Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation 2012; 84 (3): 261–268. DOI: 10.1016/j.diff.2012.06.003.
- Satoh M. Histogenesis and organogenesis of the gonads in human embryos. Med Electron Microsc 1991; 27 (3-4): 254–256. DOI: 10.1007/bf02349668.
- McKay DG, Hertig AT, Adams EC, Danziger S. Histochemical observations on the germ cells of human embryos. Anat Rec 1953; 117 (2): 201–219. DOI: 10.1002/ar.1091170206.
- Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortés L, et al.. SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech Dev 2000; 91 (1-2): 403–407. DOI: 10.1016/s0925-4773(99)00307-x.
- Yao HH-C. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol 2005; 230 (1-2): 87–93. DOI: 10.1016/j.mce.2004.11.003.
- Berta P, Hawkins JB, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, et al.. Genetic evidence equating SRY and the testis-determining factor. Nature 1990; 348 (6300): 448–450. DOI: 10.1038/348448a0.
- Rey RA, Grinspon RP. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract Res Clin Endocrinol Metab 2011; 25 (2): 221–238. DOI: 10.1016/j.beem.2010.08.013.
- McElreavey K, Barbaux S, Ion A, Fellous M. The genetic basis of murine and human sex determination: a review. Heredity (Edinb) 1995; 75 (6): 599–611. DOI: 10.1038/hdy.1995.179.
- Vilain E, McCabe ERB. Mammalian Sex Determination: From Gonads to Brain. Mol Genet Metab 1998; 65 (2): 74–84. DOI: 10.1006/mgme.1998.2749.
- Sinisi AA, Pasquali D, Notaro A, Bellastella A. Sexual Differentiation. Encyclopedic Dictionary of Genetics, Genomics and Proteomics 2003; 26 (3 Suppl): 473–494. DOI: 10.1002/0471684228.egp11478.
- Biason-Lauber A, Chaboissier M-C. Ovarian development and disease: The known and the unexpected. Semin Cell Dev Biol 2015; 45 (59-67): 59–67. DOI: 10.1016/j.semcdb.2015.10.021.
- Rey R, Lukas-Croisier C, Lasala C, Bedecarrás P. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 2003; 211 (1-2): 21–31. DOI: 10.1016/j.mce.2003.09.007.
- Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J Obstet Gynaecol Res 2010; 36 (5): 929–937. DOI: 10.1111/j.1447-0756.2010.01272.x.
- Barteczko KJ, Jacob MI, Jacob MI. Development, Shape and Fate of Gubernaculum Hunteri and Processus Vaginalis Peritonei - Own Phases of Testicular Descent. Adv Anat Embryol Cell Biol 2000; 156:iii-x: 17–72. DOI: 10.1007/978-3-642-58353-7_4.
- Hawkins JR, Taylor A, Berta P, Levilliers J, Auwera B Van der, Goodfellow PN. Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal. Hum Genet 1992; 88 (4): 471–474. DOI: 10.1007/bf00215684.
- Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Mol Cell Endocrinol 2012; 354 (1-2): 34–53. DOI: 10.1016/j.mce.2011.09.035.
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003; 4 (12): 969–980. DOI: 10.1038/nrg1225.
- Cunha GR. The dual origin of vaginal epithelium. Am J Anat 1975; 143 (3): 387–392. DOI: 10.1002/aja.1001430309.
- Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. J Pediatr 1975; 86 (3): 395–398. DOI: 10.1016/s0022-3476(75)80969-3.
- Zalel Y, Pinhas-Hamiel O, Lipitz S, Mashiach S, Achiron R. The development of the fetal penis-anin uterosonographic evaluation. Ultrasound Obstet Gynecol 2001; 17 (2): 129–131. DOI: 10.1046/j.1469-0705.2001.00216.x.
- Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, et al.. The Role of Sonic Hedgehog-Gli2 Pathway in the Masculinization of External Genitalia. Endocrinology 2011; 152 (7): 2894–2903. DOI: 10.1210/en.2011-0263.
- BASKIN LS, LEE YT, CUNHA GR. Neuroanatomical ontogeny of the human fetal penis. BJU Int 1997; 79 (4): 628–640. DOI: 10.1046/j.1464-410x.1997.00119.x.
- Kaplan GW, Lamm DL. Embryogenesis of Chordee. J Urol 1975; 114 (5): 769–772. DOI: 10.1016/s0022-5347(17)67140-4.
- Baskin L, Erol A, Jegatheesan P, Li Y, Liu W, Cunha G. Urethral seam formation and hypospadias. Cell Tissue Res 2001; 305 (3): 379–387. DOI: 10.1007/s004410000345.
- Kluth D, Fiegel HC, Geyer C, Metzger R. Embryology of the distal urethra and external genitals. Semin Pediatr Surg 2011; 20 (3): 176–187. DOI: 10.1053/j.sempedsurg.2011.03.003.
- Omar HA, Hummel M, Jones EA, Perkins KC. Hypoplastic external genitalia in association with X;autosome chromosome translocation. J Pediatr Adolesc Gynecol 1999; 12 (3): 161–164. DOI: 10.1016/s1038-3188(99)00011-x.
- Ferguson-Smith MA. Karyotype-phenotype Correlations in Gonadal Dysgenesis and Their Bearing on the Pathogenesis of Malformations. J Med Genet 1965; 2 (2): 142–155. DOI: 10.1136/jmg.2.2.142.
最近更新时间: 2025-09-22 08:00