1: Embryology of Urinary Tract
Este capítulo levará aproximadamente 20 minutos para ler.
Introduction
Despite the functional differences between the urinary tract and the reproductive system, the embryologic origins and development of these systems are integrated with one another. The urinary tract is comprised of the kidneys, ureters, bladder, and urethra. This system functions to excrete waste and maintain fluid and electrolyte balance. The kidneys also produce hormones that regulate blood pressure, erythropoietin production, and vitamin D synthesis. The genital system is composed of gonads, reproductive tracts, and external genitalia. This system synthesizes and receives hormones for growth and development, produces and transports spermatozoa and oocytes, and, in females, sustains and supports fetal development.
An understanding of the embryologic development of the genitourinary tract provides a foundation for understanding the anatomic and physiologic function of these organs as well as the pathophysiology of maldeveloped structures. While embryology pertaining to various genitourinary conditions are provided in detail in other chapters, the purpose of this chapter is to provide an overview of the embryology for the genitourinary tract.
In this overview, we use “male” to refer to the internal and external genitalia that are most typically seen in persons with the 46,XY genotype and “female” to refer to the internal and external genitalia that are most typically seen in persons with the 46,XX genotype.
Kidneys and Upper Urinary Tract
Kidney and upper urinary tract development progresses through the proposed stages of the pronephros, mesonephros, and metanephros (Figure 1) The development of each renal unit ultimately gives rise to the functional renal parenchyma (comprised of about 250,000 to 3 million nephrons1,2), and the collecting system: minor and major calyces, renal pelvis, and ureter.
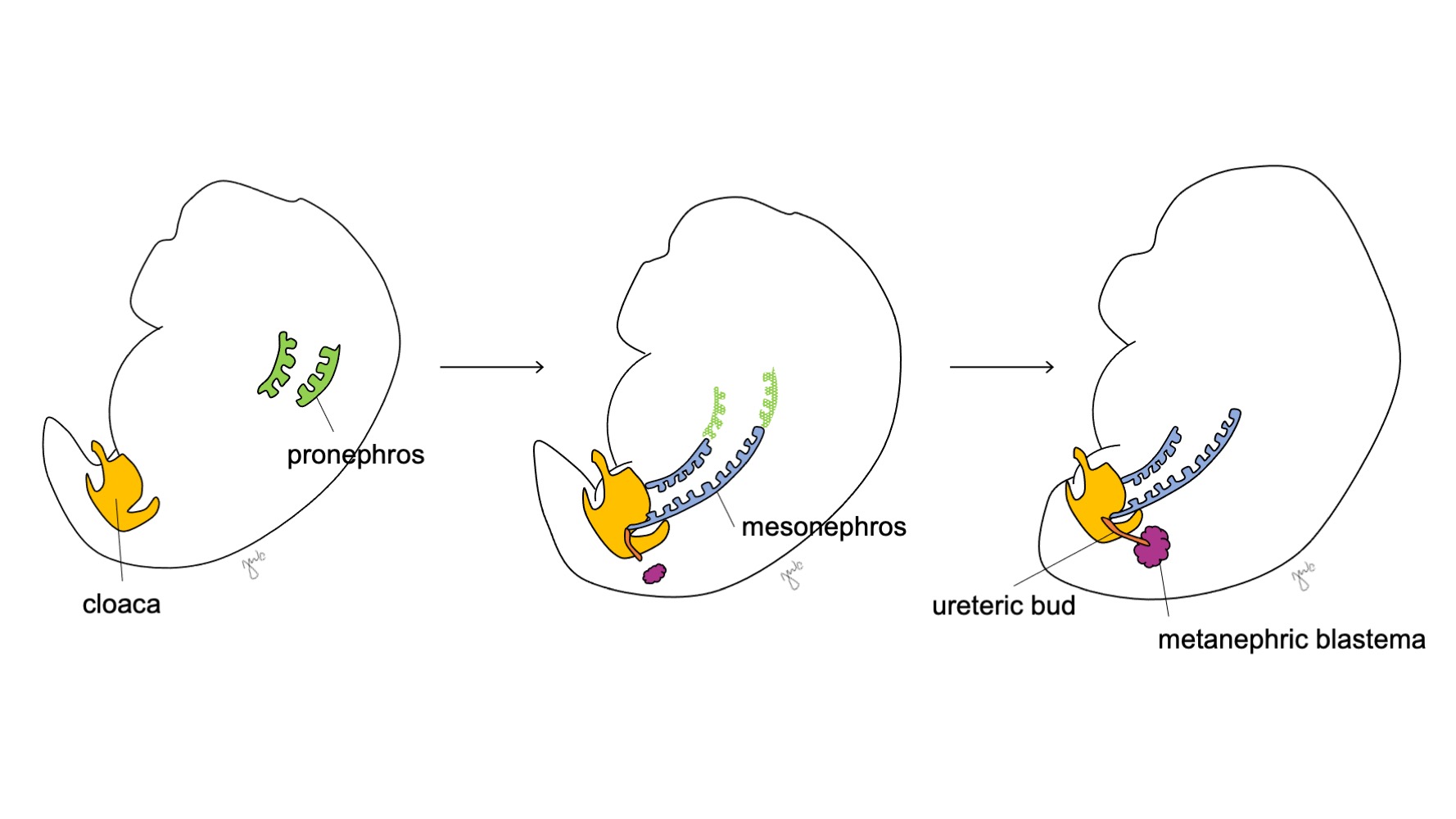
Figure 1 Developmental stages of the kidney and upper urinary tract. The kidney progresses through the pronephros at week 3 (green), mesonephros starting at week 4 (blue), and metanephros starting at week 5 of gestation. As the mesonephros attaches to the cloaca, the ureteric bud (orange) arises adjacent to this junction and fuses with the metanephric blastema (maroon).
In week 3 of gestation, intermediate mesoderm in the cervical and upper thoracic regions condenses to form the pronephros. The pronephros is a primitive and non-functional structure of hollow spheres of tissue and apparently serves a transient role in the embryologic development of the kidney. The pronephric ducts extend caudally to give rise to the mesonephric ducts and degenerate by the end of the fourth week of gestation. Through the concept that ontogeny recapitulates phylogeny (i.e. embryologic development progresses through points of species evolution), the transient role of the human pronephros is proposed through its function in the embryologic kidney of various chordates.3 However, little is known about the human pronephros and pronephric structures were not detectable on evaluation of human embryos at 3-4 weeks of development.3
The mesonephros arises at the level of the thoracic vertebra at approximately 4 weeks of gestation.3 Toward the end of the fourth week, the mesonephros extends caudally to attach to the cloaca. The mesonephros functions as a primitive kidney that filters blood through tubules. Although tubules degenerate and the mesonephros shrinks relative to the size of the growing embryo, the mesonephric (Wolffian) ducts persist bilaterally and contribute further to urinary tract development (bladder trigone and ureters) and the male reproductive tract (vas deferens, epididymis, seminal vesicle, appendix epididymis).4
After the mesonephros attaches to the cloaca, the ureteric bud arises from the distal posterior mesonephric duct near its junction with the cloaca at week 5 of gestation.3 The metanephric blastema concurrently arises as a mass of mesenchymal tissue aggregated from intermediate mesoderm. Together, the ureteric bud and metanephric blastema form the metanephros, which is the final stage in kidney and upper tract development. The metanephric blastema and ureteric bud co-induce one another to develop: the metanephric blastema encapsulates and fuses with the ureteric bud and the ureteric bud penetrates the metanephric blastema and starts bifurcating. As the ureteric bud elongates to form the ureter, it bifurcates to form the renal pelvis and major calyces, which then branch to form minor calyces, the renal pyramid, and ultimately the collecting tubes of the nephron (Figure 2) Meanwhile, as the ureteric bud elongates and bifurcates, the metanephric blastema forms nephron structures (Figure 3) Vascular endothelial growth factor-2 (VEGF-2) initiates vascularization at the metanephric blastema.5 Excretory S-shaped tubules acquire capillaries to form glomeruli that each have an encapsulating Bowman’s capsule. These excretory tubules lengthen to form the proximal convoluted tubule, loop of Henle, and distal convoluted tubule. The distal convoluted tubule joins with the collecting duct (from the ureteric bud) to form the nephron.
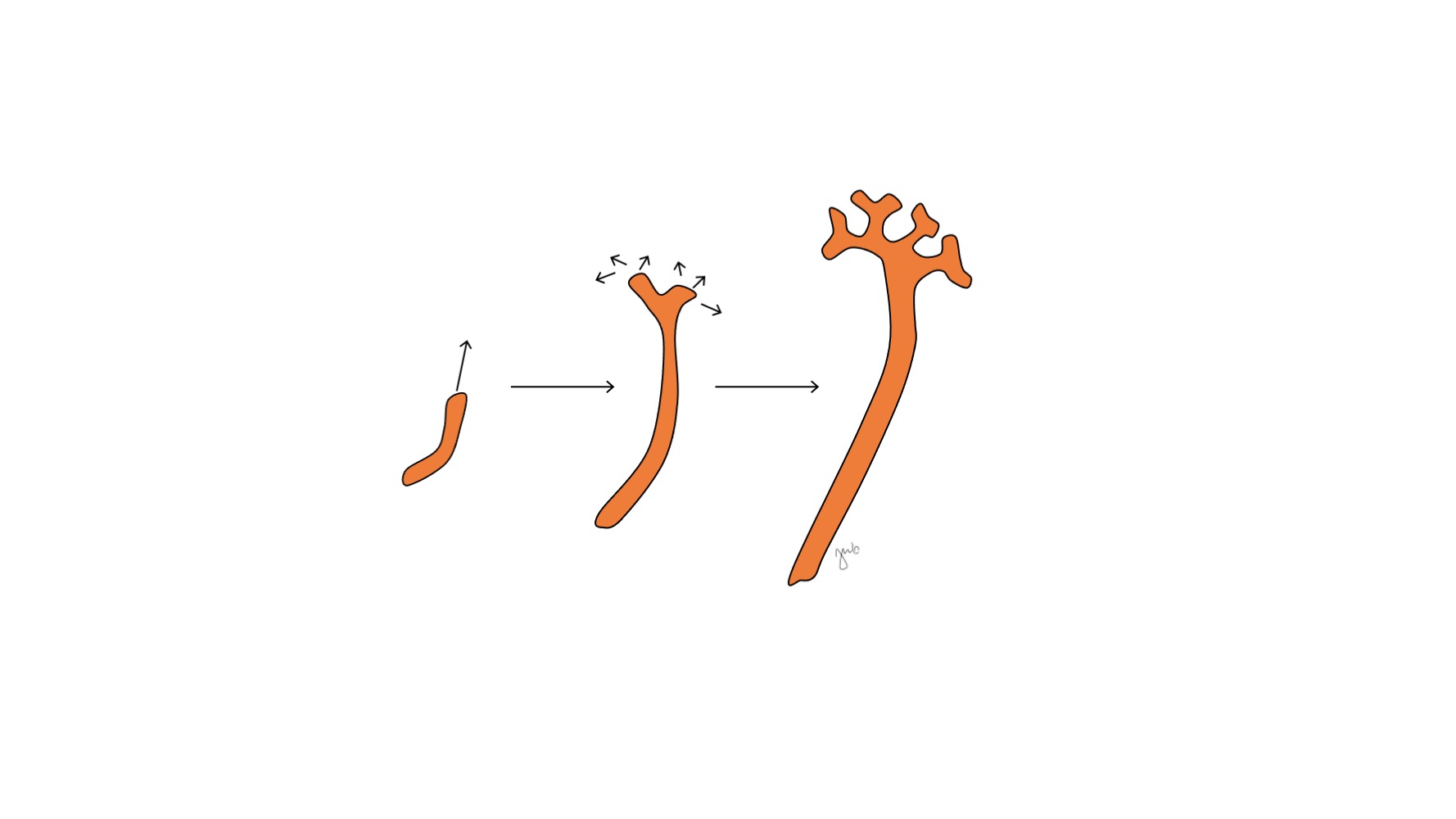
Figure 2 Ureteric bud. The ureteric bud elongates and branches to form the renal pelvis, major and minor calyces, and collecting duct of the nephron.
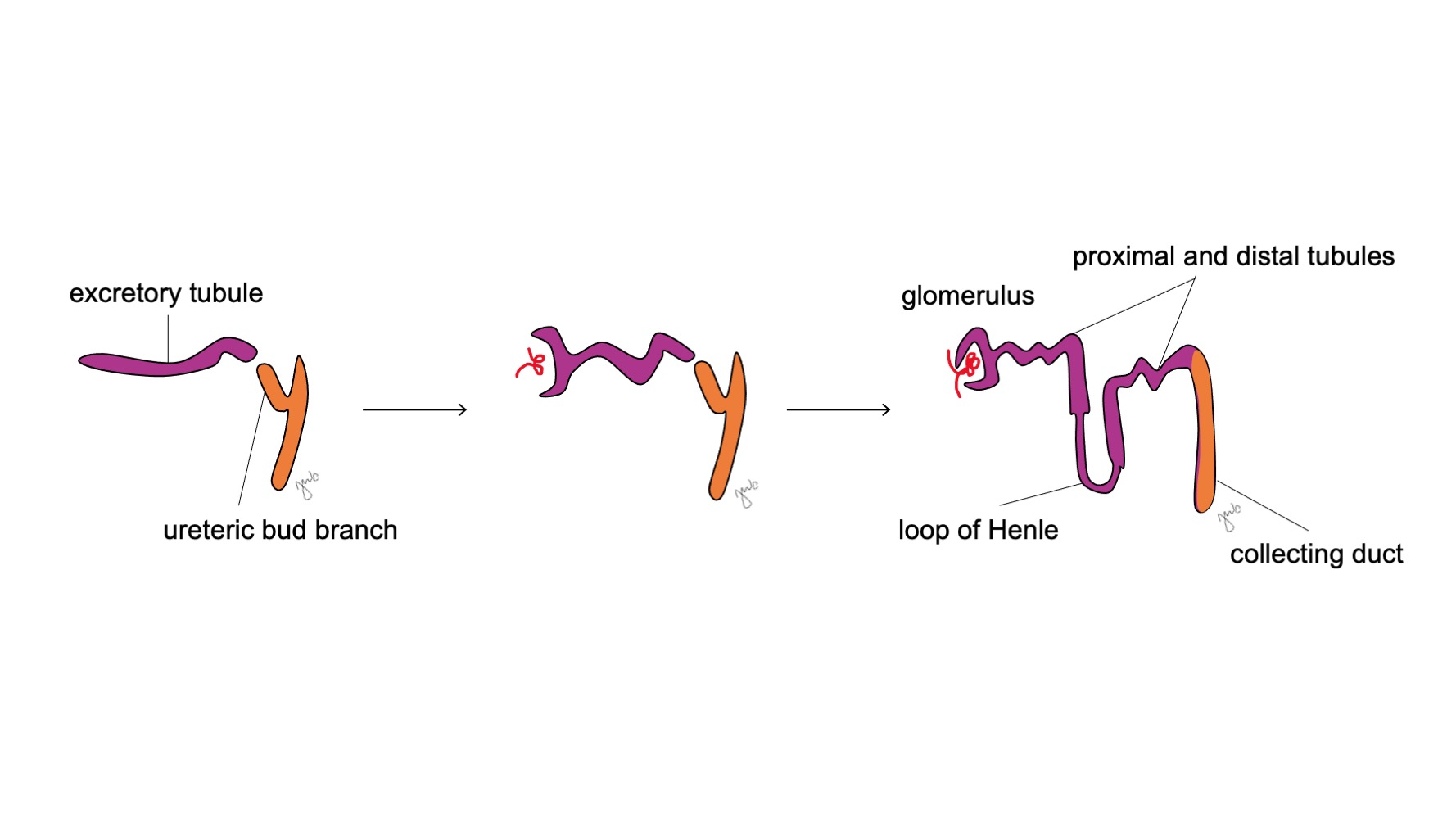
Figure 3 Nephron development. Excretory S-shaped tubules (purple; from metanephric blastema) acquire capillaries to form glomeruli, elongate to form the proximal and distal tubules and loops of Henle, and join with collecting ducts from the ureteric bud (orange; from mesonephric ducts) to ultimately form nephrons.
The ureteric bud and metanephric blastema reciprocally induce growth through various molecular factors and interactions that contribute to the development of the metanephros. Bone morphogenetic protein BMP-4 is expressed in the mesenchyme and serves dual functions to inhibit ureteric budding and promote ureteral elongation.6 Local expression of BMP-antagonist gremlin (grem1) allows for ureteral budding to initiate.6,7 After ureteric budding initiation, BMP-4 subsequently promotes elongation and appears to be involved in promoting the development of periureteral smooth muscle.6,8 BMP-7, from the metanephric blastema and ureteric bud, maintains and sensitizes nephron progenitors to ureteric bud signaling.6 The metanephric blastema secretes glial-cell derived neurotrophic factor (GDNF) that also induces the ureteric bud to branch and elongate.9 Glycoprotein WNT11 is expressed at the tip of the branching ureteric bud and helps maintain expression of GDNF.10,11 There is reciprocal interaction between WNT11, GDNF, and the RET tyrosine kinase receptor in metanephros development as WNT11 and RET mutations result in branching defects and renal hypoplasia.11 Fibroblast growth factor (FGF) ligands and their corresponding receptors (FGFR) also contribute to metanephric mesenchymal patterning, induction of the ureteric bud, ureteric bud branching, and nephrogenesis.12 Furthermore, direct contact with mesenchyme also promotes ureteric bud elongation and branching pattern.13
The metanephros develops into the functional and anatomically positioned kidney with early urine production, renal ascension and revascularization, and renal rotation. Fetal urine production starts at week 10-11 of gestation and becomes the predominant component of amniotic fluid.14 The kidneys are initially in close proximity to each other in the sacral region of the embryo.15,16 As the embryo grows longitudinally, each kidney ascends at weeks 6-9 from its initial pelvic location to the upper retroperitoneum at the lower thoracic and upper lumbar region (Figure 4) Transient blood vessels develop and degenerate as the kidneys ascend until they reach their final destination in the upper retroperitoneum and the definitive renal arteries and veins develop. The kidneys rotate so that the renal pelvis moves from an anterior to medial orientation.16
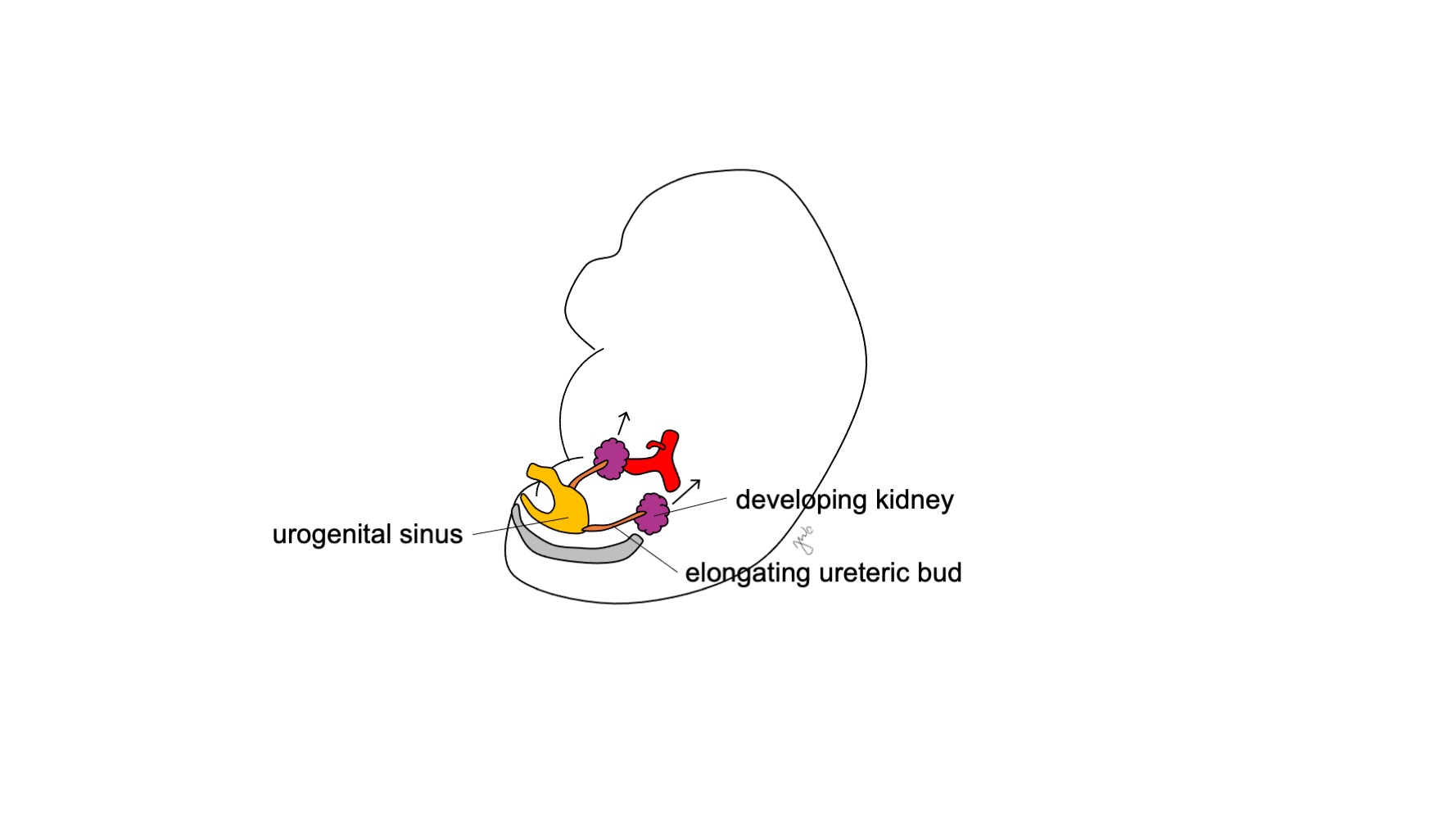
Figure 4 Renal ascent. The developing kidney ascends from its pelvic location to the upper retroperitoneum.
Key Points
The developing kidney and upper urinary tract develop through the pronephros, mesonephros, and metanephros stages. The metanephros is comprised of the metanephric blastema and ureteric bud that respectively develop into the renal parenchyma and collecting system. The mesonephros persists and contributes to the development of the bladder trigone, upper tract collecting system, and the male reproductive system.
Lower Urinary Tract
The cloaca acts as a common chamber for the urogenital and anorectal tracts as it connects to the allantois (yolk sac) and the hindgut. It is a combination of ectoderm lined by endoderm that opens at a single orifice at the caudal aspect of the developing embryo. As the mesonephros attaches to the cloaca and the upper urinary tract develops as the metanephros, the lower urinary tract develops from the cloaca to form the bladder and urethra.
At approximately week 7 of gestation, the urorectal septum grows caudally, separating the cloaca into the anterior urogenital sinus and the posterior anorectal canal, and ultimately becomes the perineal body (Figure 5)17 The urogenital sinus then subdivides cranially and caudally to form the respective bladder and urethra.
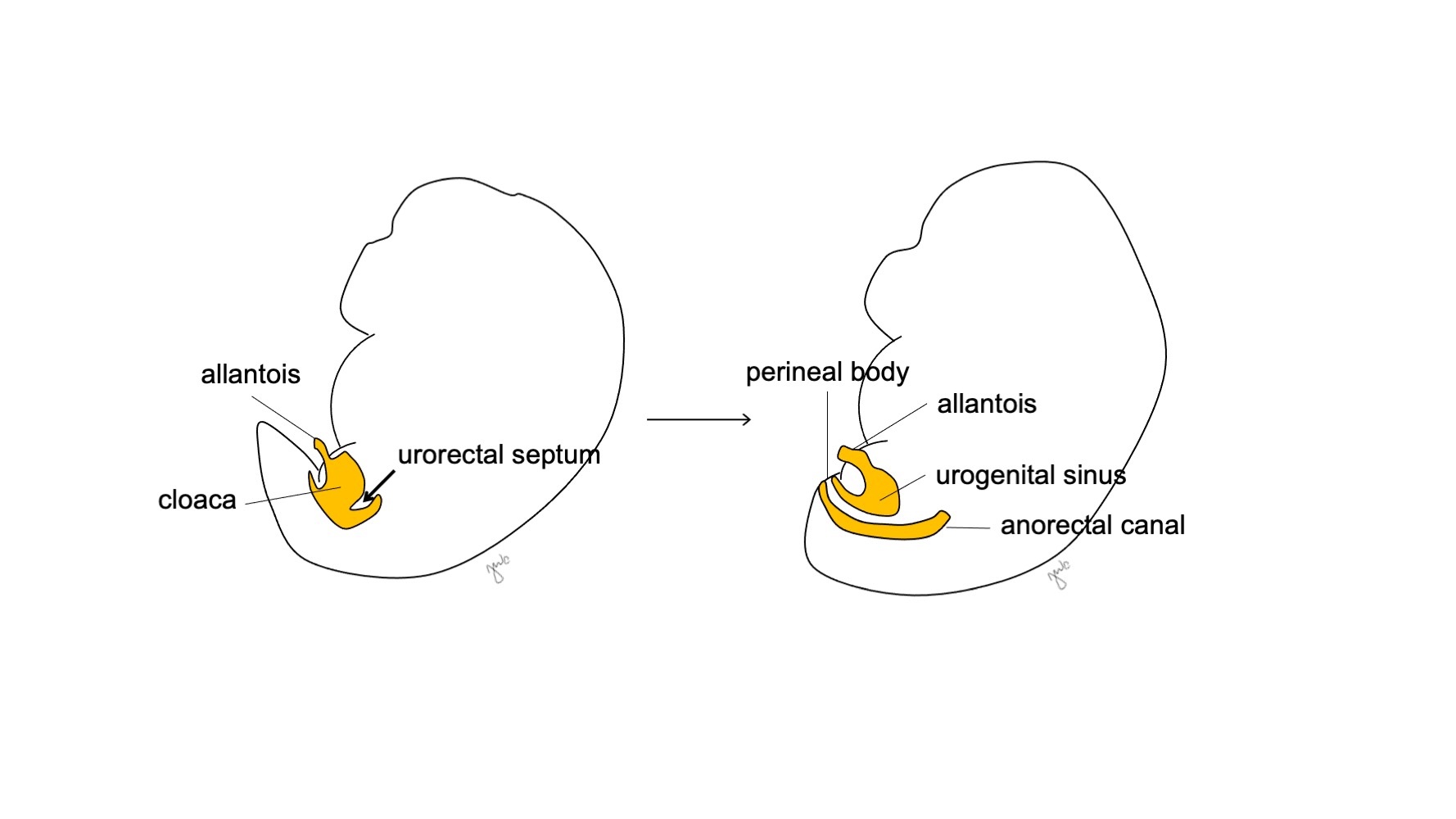
Figure 5 Cloacal division. The urorectal septum divides the cloaca into the urogenital sinus and the anorectal canal. The urorectal septum then develops into the perineal body.
The cranial aspect of the urogenital sinus enlarges and becomes the bladder that is initially continuous with the allantois (Figure 6) While the endoderm of the former cloaca gives rise to the urothelial lining of the bladder.18 differentiation of bladder smooth muscle relies upon mesenchymal-epithelial interactions.19 The mesonephric duct that had previously attached to the cloaca remains attached to the developing bladder; it regresses and is absorbed caudally into the bladder trigone and ureteral orifices. Vitamin A-induced apoptosis of the mesonephric ducts, interactions between the bladder and ureteral smooth muscle, and endodermal origins are other proposed mechanisms contributing to trigonal development.20,21,22
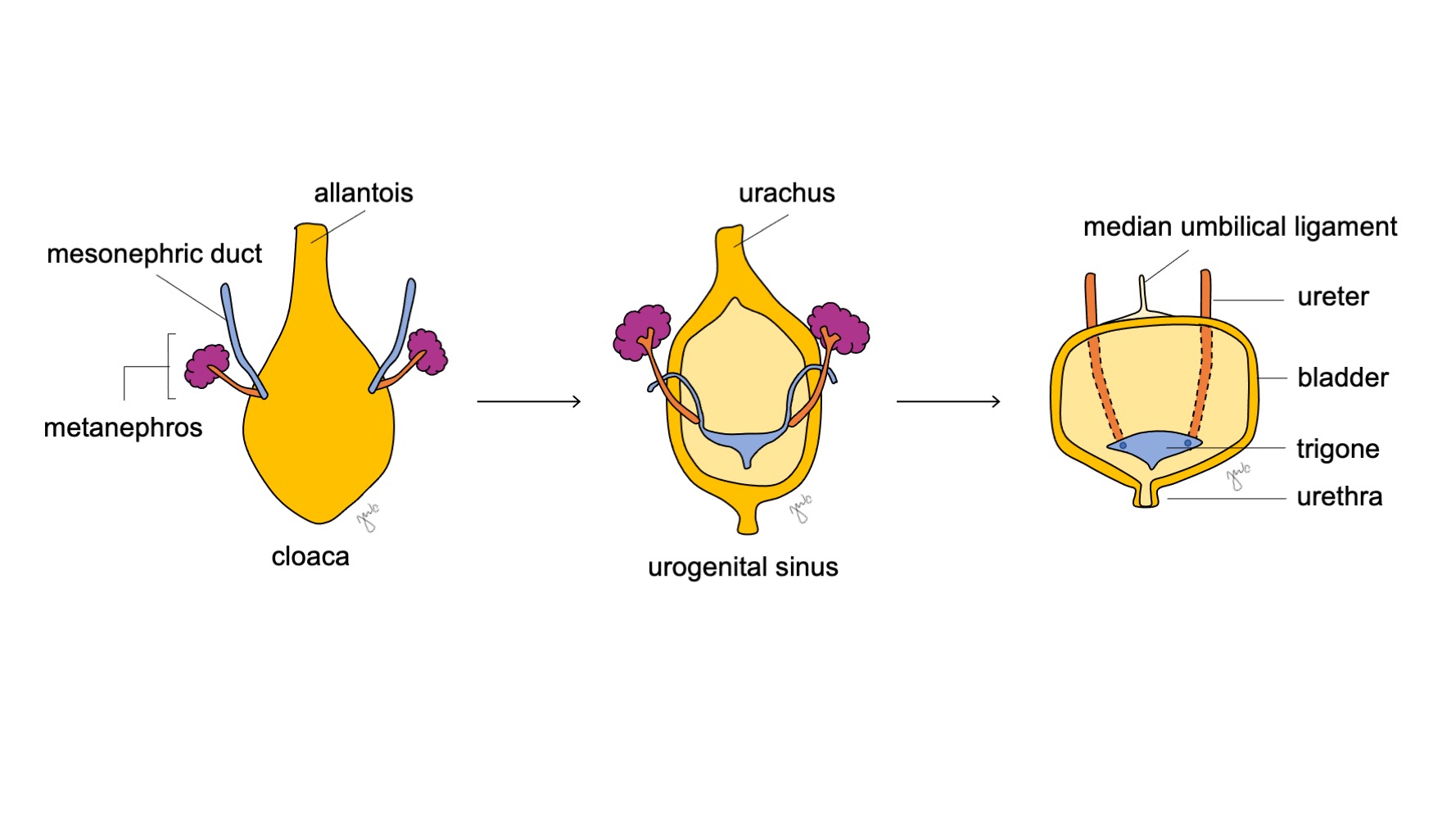
Figure 6 Bladder development. The cranial aspect of the urogenital sinus enlarges to become the bladder. The mesonephric duct (blue) regresses and is absorbed into the bladder trigone and ureteral orifices. Meanwhile, the allantois involutes and becomes the urachus, which then becomes more fibrotic as the median umbilical ligament.
The remainder of the mesonephric ducts subsequently develop into the male reproductive tract (discussed in the gonads and reproductive tracts section). Products of the mesonephric ducts move further apart as the embryo continues to grow: the ureteral orifices move craniolaterally as the male reproductive tracts move caudally and medially. Displacement of the ureteric bud relative to the location of the mesonephric duct can affect the ultimate location of the ureteral orifice and the development of the respective kidney.23,24 Milder displacement can result in a craniolateral or caudomedial position of the ureteral orifice whereas severe displacement can result in an ectopic ureter inserting outside of the bladder and/or renal dysplasia.23,24
As the bladder develops, the allantois involutes and becomes the urachus that connects the dome of the bladder with the umbilicus. After birth, the urachal remnant becomes more fibrotic and becomes the median umbilical ligament.25
The inferocaudal aspect of the urogenital sinus forms the prostatic and membranous urethra in males and urethra and vaginal vestibule in females.25 Similar to the bladder, the urothelial lining of the urethra is derived from endoderm.18 In males, the urogenital sinus extends to the genital tubercle to form the prostatic and membranous urethra whereas its associated urogenital folds form the penile urethra.18,26 Further branching and outgrowths from the prostatic urethra form the prostatic glands at approximately 9-10 weeks of gestation.27 The prostate stroma arises from the mesenchyme and develops under the influence of androgens and paracrine signaling from multiple factors, including FGF, sonic hedgehog (SHH), transcription factor Nkx3.1, homeobox (HOX) genes, forkhead box (FOX) transcription factors, SRY-box transcription factor (SOX-9), BMP, and WNT.27,28,29,30,31 While testosterone can contribute to prostatic development, dihydrotestosterone (DHT) has a much more potent effect on the prostate.27 Prostatic mesenchyme forms stromal tissue and the smooth muscle surrounding prostatic glands and ducts. The bulbourethral (Cowper’s) glands in males form by budding from the urethra. The penile urethra is formed when the urethral plate tubularizes18,26 (discussed in the external genitalia section). Development of the glanular portion of the urethra is debated at this time and may form from tubularization with subsequent endodermal differentiation into squamous epithelium based on more recent studies.18,32,33
In females, the urogenital sinus forms the entire urethra and the distal vagina. Homologous to the male prostatic glands, the cranial aspect of the urethra forms the paraurethral (Skene’s) glands. Homologous to the male bulbourethral (Cowper’s) glands, Bartholin’s glands form by budding from the urethra.
Key Points
The cloaca is initially a common chamber that is divided by the urorectal septum to form the urogenital sinus and the anorectal canal. The urogenital sinus subsequently enlarges cranially to form the bladder and contributes to the urethra in both sexes. The allantois concurrently involutes to form the urachus, which later becomes the median umbilical ligament.
Gonads and Reproductive Tracts
The gonads and internal reproductive tract of male and female sexes are initially undifferentiated. Influence from genetic and hormonal factors determine development to form either the testes and associated mesonephric (Wolffian) structures or ovaries and associated paramesonephric (Müllerian) structures.
During week 5 of gestation, proliferation and condensation of the germinal epithelium and mesenchyme form the gonadal ridge adjacent to the mesonephros. Primordial germ cells are incorporated with clusters of epithelium called sex cords. The sex cords invaginate local mesenchymal tissue and enable migration of the primordial germ cells through yolk sac cavity to the gonadal ridge at 6 weeks.34 As germ cells and sex cords cluster together at the gonadal ridge, cortical and medullary regions of the gonad develop.35,36 Factors WNT4 and SOX-9 are expressed at the gonadal ridges and vary in their expression in human sex determination.37 Gonad formation is also regulated by transcription factors including Wilms tumor 1 (WT1), LIM homeobox 9 (LHX9), and steroidogenesis factor 1 (SF1).38
The undifferentiated gonads are bipotential (Figure 7) and the primordial germ cells have the ability develop into spermatogonia in males or oogonia in females until the seventh week of gestation.38 Mesonephric (Wolffian) ducts and paramesonephric (Müllerian) ducts are also bipotential at this point.
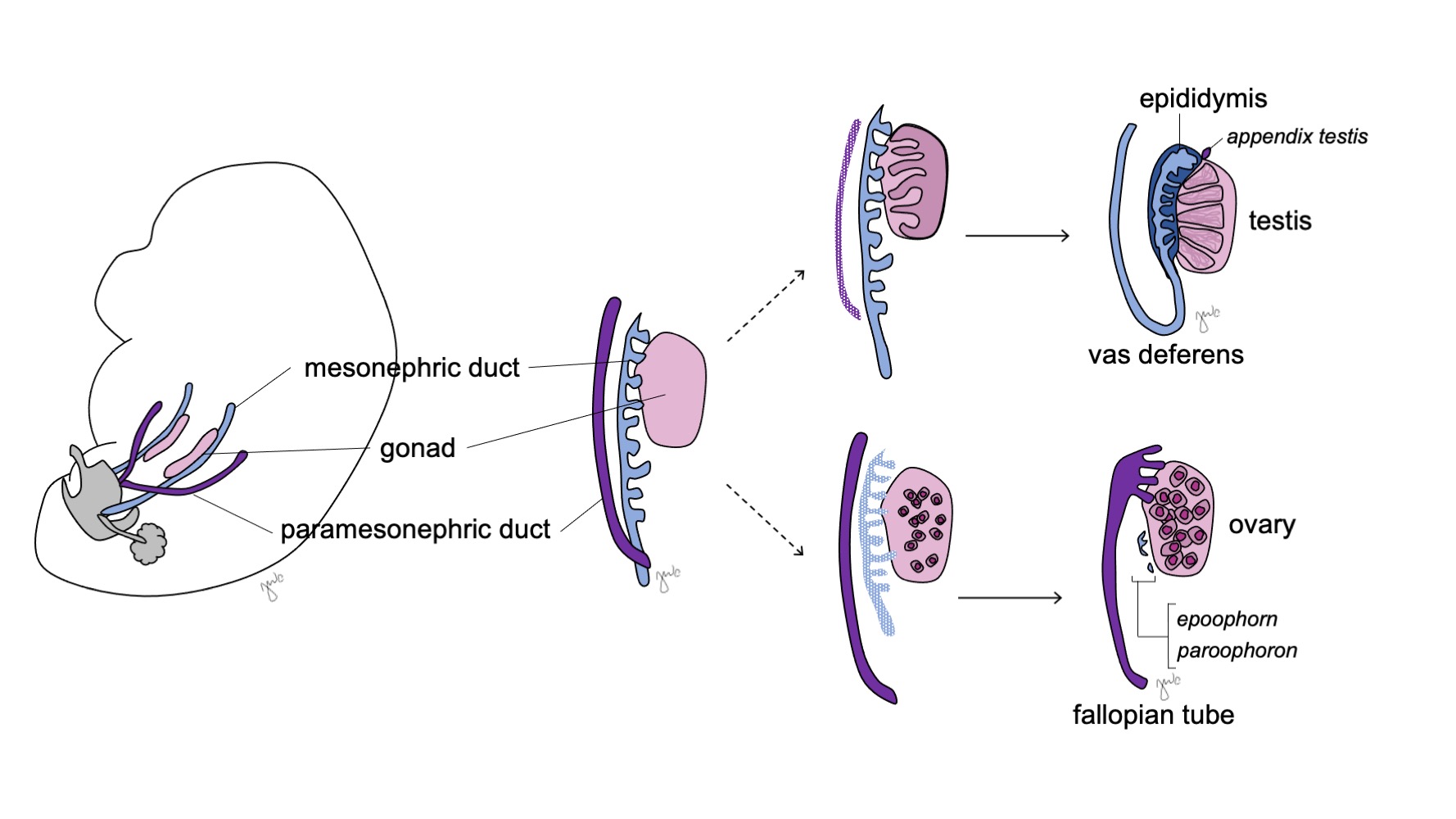
Figure 7 Gonadal differentiation. The gonad (pink) typically differentiates into either the testis with corresponding mesonephric duct structures (blue) or the ovary with corresponding paramesonephric duct structures (purple).
The Male Gonad and Reproductive Tract
In males, the undifferentiated gonad develops into the testis and the mesonephric (Wolffian) ducts develop into the epididymis, appendix epididymis, vas deferens, seminal vesicle, and ejaculatory duct.
The SRY (sex-determining region of the Y-chromosome) gene (also known as testis-determining factor [TDF]) is located on the short arm of the Y chromosome at Yp11.3 and initiates male sex differentiation.39,40 The corresponding SRY protein and transcription factors from SOX-9, DAX-1, WT1, and SF1 genes influence gonadal differentiation,.% cite mcelreavey1995a vilain1998a –file 01-01 %} SRY has been found to upregulate SOX-9 to activate expression of SF1 and promote the differentiation of Sertoli and Leydig cells.37,41,42 The primitive gonadal sex cords organize into the rete testis and seminiferous cords. Sertoli cells and Leydig cells develop between the seminiferous cords and initiate hormone production that promote male differentiation (discussed below). The tunica albuginea encapsulates these cords to form the testicle. The seminiferous cords eventually develop a lumen and become the seminiferous tubules at puberty.
Sertoli and Leydig cells subsequently begin testicular hormone production that results in a cascade of masculinization processes. Sertoli cells secrete Müllerian-inhibiting factor (MIF) or anti-Müllerian hormone (AMH) under the influence of SOX-9, SF1, WT1, and DAX1 as well as follicle-stimulating hormone (FSH).43 Anti-Müllerian hormone subsequently causes the degeneration of the paramesonephric (Müllerian) ducts, with remnants being the appendix testis and prostatic utricle.40,43,44 SOX-9 and SF1 interact to increase the effect of AMH to cause Müllerian regression. Leydig cells concurrently respond to luteinizing hormone (LH) and analogously structured human chorionic gonadotropin (hCG) to initiate testosterone production at week 8-9. Testosterone induces the differentiation of the mesonephric duct (Figure 8) into the epididymis, appendix epididymis, vas deferens, ejaculatory duct, and seminal vesicle44 as well as masculinization of the external genitalia through conversion to DHT.
Following development, testicular descent starts at week 10 and depends on intra-abdominal pressure, hormonal influences, and tension from the gubernaculum.45 As the testis enters the deep (internal) inguinal ring and forms the inguinal canal, a layer of peritoneum forms the tunica vaginalis. The testis passes the inguinal canal between weeks 20-28 and exits through the superficial (external) inguinal ring to enter the scrotum. The external oblique muscle, internal oblique muscle, and transversalis fascia of the anterior abdominal wall provide the respective scrotal layers that cover the testis as the external spermatic fascia, cremasteric fascia and muscle, and internal spermatic fascia.45
The Female Gonad and Reproductive Tract
In females, the undifferentiated gonad develops into the ovary and the paramesonephric (Müllerian) duct develops into the fallopian tube, uterus, cervix, and proximal vagina.
Ovaries develop in the absence of the SRY gene in those with 46,XX genotypes or with 46,XY genotypes but loss of the SRY gene.46 The ovarian cortex proliferates and makes primary sex cords that extend to the gonadal medulla. At 4 months, the cords divide and form primordial follicles comprised of oogonia and follicular cells. Oogonia undergo mitosis and primary oocytes are arrested at prophase I at the time of birth as a reserve of follicles for future reproduction.42 Compared to testicular descent, the ovaries descend into the pelvis in a shorter amount of time.45
Female gonadal development has been previously considered to be the “default” mechanism that occurs from no SRY gene influence. However, ovarian development does not appear to be a simple passive process. It is actively promoted through the genetic and molecular influences of WNT4 and DAX-1.37,40,41,42 Both genes have anti-testis effects and WNT4 has been considered the “ovary-determining gene”.37,40 WNT4 upregulates DAX-1 expression to suppress SF1 transcriptional activity, inhibit function of SOX-9, and ultimately promote ovarian development.41,42
Prior to sexual differentiation, the paramesonephric (Müllerian) ducts arise as epithelial invaginations lateral to the mesonephric (Wolffian) ducts.47 The absence of AMH early in embryonic development allows the paramesonephric (Müllerian) ducts to develop into the fallopian tubes, uterus, cervix, and proximal vagina (Figure 8).42,44,48 The lateral aspects of these ducts form the fallopian tubes. The medial aspects of the paramesonephric (Müllerian) ducts fuse at the midline to form the uterus and the distal end of the combined ducts contacts the urogenital sinus to join the proximal and distal vagina (Figure 9).44,47,49 The surrounding mesenchyme gives rise to the endometrium and myometrium of the uterus.48 Homologous to the paramesonephric (Müllerian) ducts degenerating into the appendix testis in males, the mesonephric ducts degenerate in the female to form the epoophoron, paroophoron, and Gartner’s duct adjacent to the vagina.44
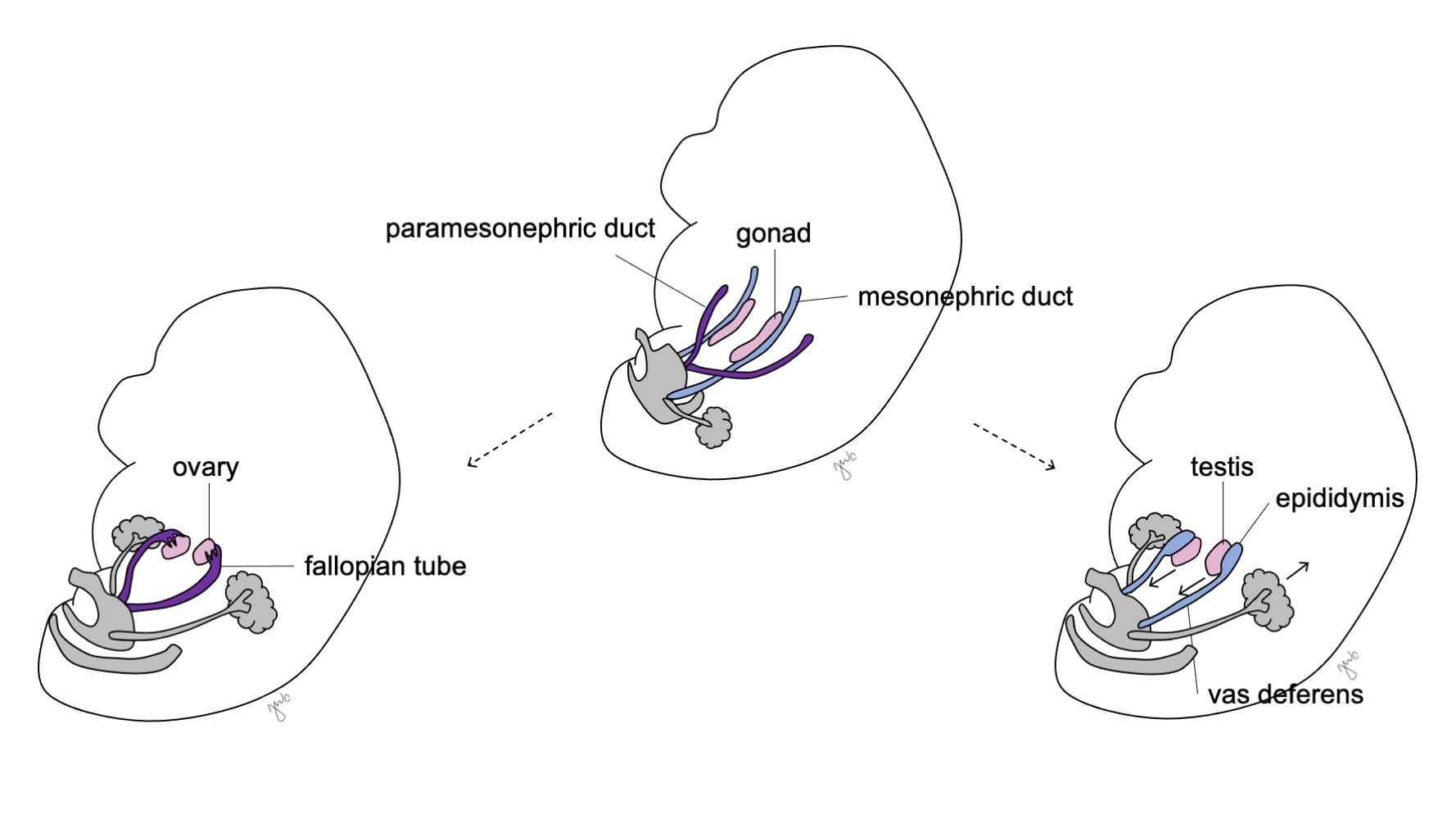
Figure 8 Mesonephric and paramesonephric duct differentiation. In males, the mesonephric duct (blue) differentiates into the epididymis, vas deferens, ejaculatory duct, and seminal vesicle. In females, the paramesonephric duct (purple) differentiates into the fallopian tube, uterus, cervix, and proximal vagina.
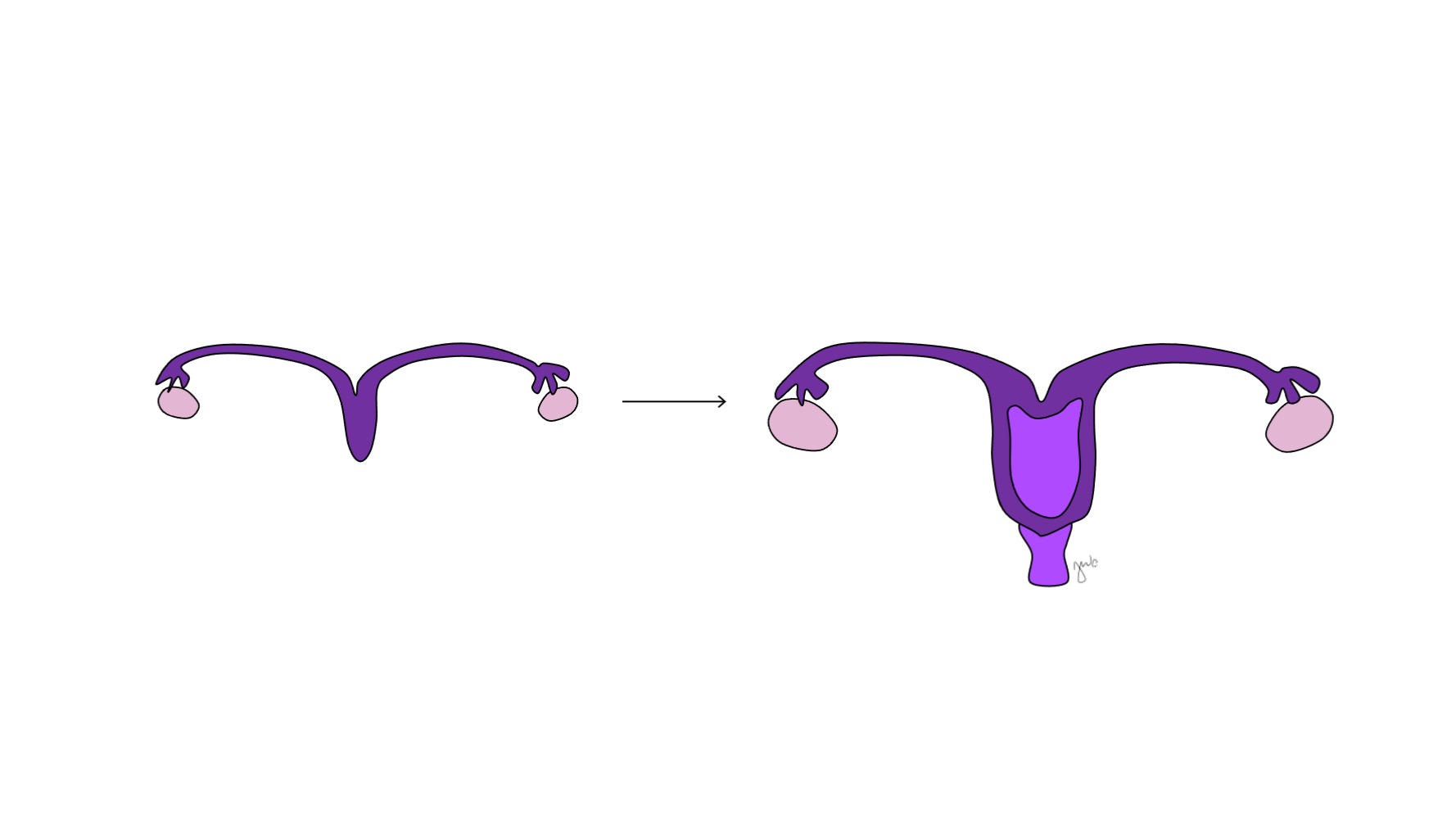
Figure 9 Paramesonephric duct fusion. The medial aspect of the paramesonephric ducts fuse at the midline to form the uterus. The distal end of this combination forms the cervix and proximal vagina, which then connects caudally with the urogenital sinus.
Comparable to gonadal development, female reproductive tract development is also not a passive “default” mechanism as WNT4 signaling is essential for Müllerian duct development and differentiation as well as suppressing male differentiation.42,48 Homeobox genes contribute to the anterior-posterior alignment of paramesonephric (Müllerian) structures.48 Similar to Sertoli cells of the testis, ovarian granulosa cells eventually secrete AMH, but do so later in fetal development after paramesonephric (Müllerian) structures have developed.43
Key Points
Gonadal and ductal differentiation is outlined in Table 1. The undifferentiated gonad and primordial germ cells can develop into the testis with spermatogonia or the ovary with oogonia. The subsequent hormone secretion from these gonads influences the mesonephric (Wolffian) duct to become the epididymis, appendix epididymis, vas deferens, ejaculatory duct, and seminal vesicle or the paramesonephric (Müllerian) duct to become the fallopian tube, uterus, cervix, and proximal vagina.
External Genitalia
The external genitalia are initially bipotential for the two sexes until the seventh week of gestation. As the cloaca is undergoing separation into the urogenital sinus and anorectal canal (previously discussed in the lower urinary tract section), folds develop on both sides of the cloaca. After the urorectal septum completes cloacal division, the lateral folds become the urogenital folds and the anterior portion of the folds coalesce to form the genital tubercle. Swellings lateral to the genital tubercle form the labioscrotal swellings. The genital tubercle, urogenital folds, and labioscrotal swellings (Figure 10) differentiate under hormonal influence from the concurrently developing gonads.
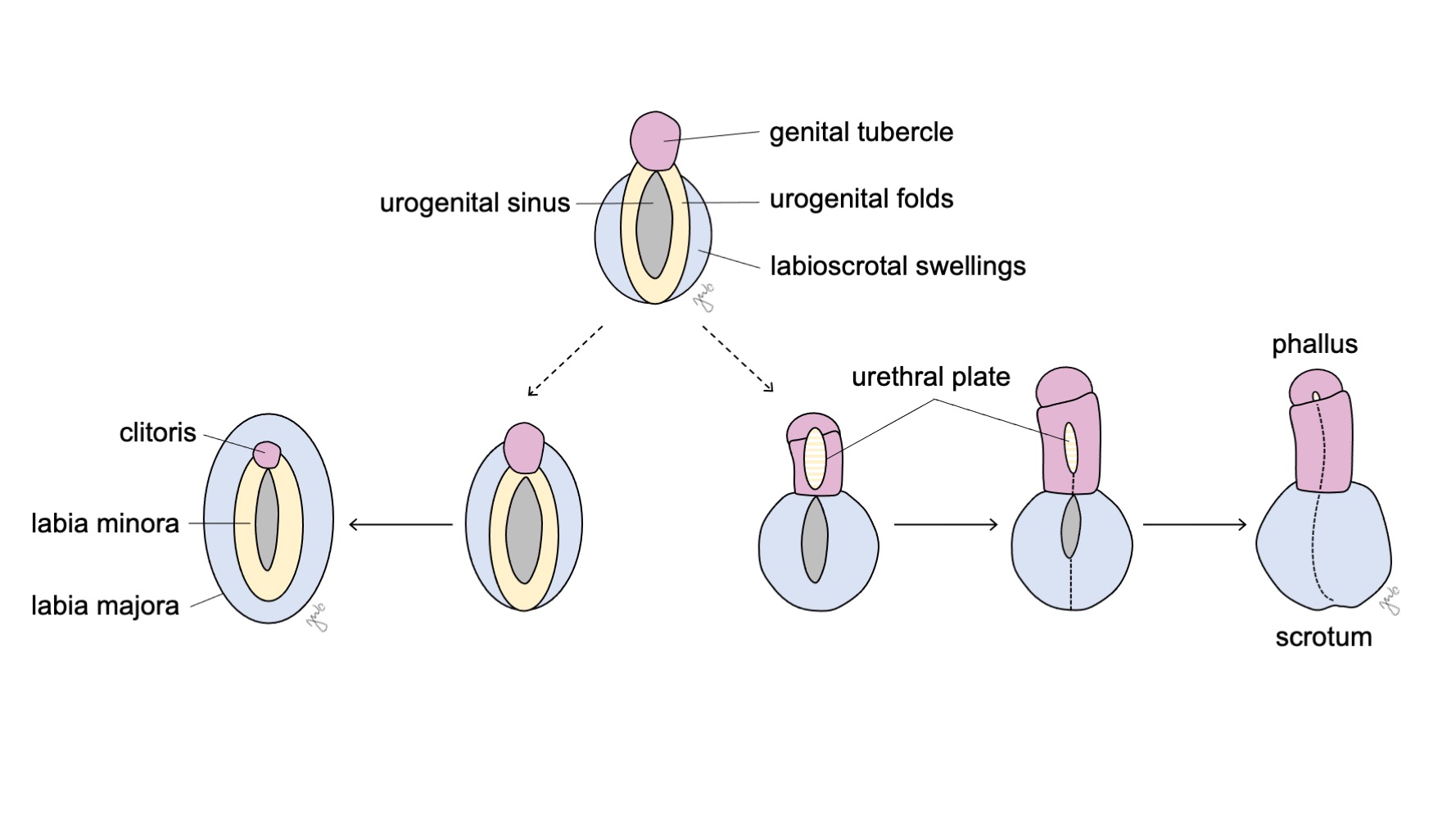
Figure 10 External genitalia differentiation. The genital tubercle (pink), urogenital folds (yellow), and labioscrotal swellings (blue) respectively differentiate either into the clitoris, labia minora, and labia majora in females or the phallus, urethral plate, and scrotum in males.
External Male Genitalia
Androgen influence from testosterone and dihydrotestosterone (DHT) causes masculinization of the urogenital sinus and external genitalia into the penis and scrotum (Figure 10).34,44
Androgen exposure causes the genital tubercle to elongate at 8-12 weeks with subsequent linear growth starting at 14 weeks of gestation and ultrasonographic detection of the phallic structure starting at 18 weeks.34,50,51 SHH is expressed in the genital tubercle and urethral plate epithelium and may facilitate androgen influence on penile formation and other forms of genital masculinization.34,52 Penile mesenchymal condensations form the corpora cavernosa and spongiosum.53 Ventral curvature of the penis has been identified in fetuses through the 20th week of gestation with gradual resolution through the remainder of gestation.54
The urogenital folds fuse in the midline to form the urethral plate as part of development of the penile urethra. A groove within the midline of the urethral plate deepens and the epithelial edges of the plate fuse to form a tube.33,55 The epithelial seam formed from this fusion is then remodeled and replaced by mesenchymal cells.55 While the proximal penile urethra fuses caudally with the prostatic urethra from the urogenital sinus, tubularization progresses distally and ventrally. As previously discussed, the development of the glanular urethra is debated. While this aspect of the urethra was previously attributed to ectodermal invagination from the tip of the glans penis, more recent studies have proposed that the glanular urethra forms through tubularization with subsequent endodermal differentiation into squamous epithelium.18,32,33 As the phallus elongates and the urethral plate tubularizes, the labioscrotal swellings fuse in the midline at the median raphe to form the scrotum.56
External Female Genitalia
Although androgen absence avoids masculinization, development of the external female genitalia is not the “default” process as two X chromosomes, ovarian development, and estrogen contribute to the process. The significance of the genetic, gonadal, and hormonal influences to development can be demonstrated by hypoplastic external genitalia in Turner syndrome patients missing an X chromosome or in reports of X chromosome translocation.57,58
The urogenital sinus and external genitalia develop into the clitoris, labia majora and minora, and lower vagina under both the influence of estrogen and absence of androgens (Figure 10) The genital tubercle regresses in the absence of androgens and forms the clitoris. Urogenital sinus (initially formed after cloacal division) gives rise to the entire female urethra and the distal vagina. The distal vagina fuses cranially with the paramesonephric ducts. Central cells degrade to form the vaginal lumen and the posterior wall invaginates to form the hymen. The urogenital folds become the labia minora with minimal posterior fusion to form the posterior fourchette. The labioscrotal swellings remain unfused in the female to become the labia majora.
Table 1 Sexual differentiation and development.
| Male | Female | |
|---|---|---|
| Gonads | ||
| Primordial germ cells | Spermatogonia | Oogonia |
| Sex cords | Seminiferous cords | Primordial follicles |
| Mesonephric (Wolffian) ducts | Epididymis, appendix epididymis, vas deferens, seminal vesicle, ejaculatory duct | Gartner’s duct, epoophoron, paroophoron |
| Paramesonephric (Müllerian) ducts | Appendix testis, prostatic utricle | Fallopian tube, uterus, cervix, proximal vagina |
| Urogenital sinus | Bladder, proximal urethra, possibly the distal glanular urethra | Bladder, urethra, distal vagina |
| External genitalia | ||
| Genital tubercle | Penis | Clitoris |
| Urogenital folds | Urethral plate | Labia minora |
| Labioscrotal swellings | Scrotum | Labia majora |
Key Points
Differentiation of the external genitalia is also outlined in Table 1. The genital tubercle becomes the phallus in males and the clitoris in females. The urogenital folds become the urethral plate in males and the labia minora in females. The labioscrotal swellings develop into the scrotum in males and the labia majora in females. As part of lower urinary tract development, the urogenital sinus forms the proximal urethra and its urogenital folds form the urethral plate for the distal urethra in males. In contrast, the urogenital sinus forms the entire urethra in females as well as the distal vagina.
Conclusion
Development of the urinary and reproductive tracts are integrated through their embryologic origins and interactions. The developing kidney progresses through the pronephros, mesonephros, and metanephros stages. The interaction between the mesonephros and cloaca gives rise to the ureteric bud and metanephric blastema that ultimately develop into the kidney and upper urinary tract. The cloaca concurrently divides, and the anterior urogenital sinus develops into the bladder and contributes to the male and female urethra. The gonads, mesonephric (Wolffian) and paramesonephric (Müllerian) ducts, and external genitalia have the potential to develop along the spectrum of male and female sexual differentiation: genetic and hormonal influences respectively influence gonadal function and phenotypic appearance of the developing embryo. Various alterations to the genes, molecular interactions, or developing tissues involved in these processes can affect the long-term anatomy and function of their respective structures.
References
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol 2011; 26 (9): 1529–1533. DOI: 10.1007/s00467-011-1843-8.
- Tryggvason K, Kouvalainen K. Number of Nephrons in Normal Human Kidneys and Kidneys of Patients with the Congenital Nephrotic Syndrome. Nephron 1975; 15 (1): 62–68. DOI: 10.1159/000180493.
- Bakker BS de, Hoff MJB van den, Vize PD, Oostra RJ. The Pronephros; a Fresh Perspective. Integr Comp Biol 2019; 59 (1): 29–47. DOI: 10.1093/icb/icz001.
- Ludwig KS, Landmann L. Early development of the human mesonephros. Anat Embryol (Berl) 2005; 209 (6): 439–447. DOI: 10.1007/s00429-005-0460-3.
- Nagata M. Glomerulogenesis and the role of endothelium. Curr Opin Nephrol Hypertens 2018; 27 (3): 159–164. DOI: 10.1097/mnh.0000000000000402.
- Nishinakamura R, Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatr Nephrol 2014; 29 (4): 681–686. DOI: 10.1007/s00467-013-2671-9.
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 2004; 131 (14): 3401–3410. DOI: 10.1242/dev.01251.
- Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D. Antagonism of BMP4 Signaling Disrupts Smooth Muscle Investment of the Ureter and Ureteropelvic Junction. J Urol 2009; 181 (1): 401–407. DOI: 10.1016/j.juro.2008.08.117.
- Sajithlal G, Zou D, Silvius D, Xu P-X. Eya1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol 2005; 284 (2): 323–336. DOI: 10.1016/j.ydbio.2005.05.029.
- Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev 2004; 14 (5): 550–557. DOI: 10.1016/j.gde.2004.07.009.
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11andRet/Gdnfpathways cooperate in regulating ureteric branching during metanephric kidney development. Development 2003; 130 (14): 3175–3185. DOI: 10.1242/dev.00520.
- Walker KA, Sims-Lucas S, Bates CM. Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr Nephrol 2016; 31 (6): 885–895. DOI: 10.1007/s00467-015-3151-1.
- Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymal–epithelial contact in the developing kidney. Proc Natl Acad Sci U S A 1999; 96 (13): 7330–7335. DOI: 10.1073/pnas.96.13.7330.
- Underwood MA, Gilbert WM, Sherman MP. Amniotic Fluid: Not Just Fetal Urine Anymore. J Perinatol 2005; 25 (5): 341–348. DOI: 10.1038/sj.jp.7211290.
- Taghavi K, Kirkpatrick J, Mirjalili SA. The horseshoe kidney: Surgical anatomy and embryology. J Pediatr Urol 2016; 12 (5): 275–280. DOI: 10.1016/j.jpurol.2016.04.033.
- Friedland GW, De Vries P. Renal ectopia and fusion. Urology 1975; 5 (5): 698–706. DOI: 10.1016/0090-4295(75)90137-5.
- Kruepunga N, Hikspoors JP, Mekonen HK, Mommen GM, Meemon K, Weerachatyanukul W, et al.. Faculty Opinions recommendation of The development of the cloaca in the human embryo. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2018; 33 (6): 24–39. DOI: 10.3410/f.734193252.793568763.
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 2008; 318 (1): 143–152. DOI: 10.1016/j.ydbio.2008.03.017.
- Baskin LS, Hayward SW, Young P, Cunha GR. Role of Mesenchymal-Epithelial Interactions in Normal Bladder Development. J Urol 1996; 56 (5): 1820–1827. DOI: 10.1097/00005392-199611000-00101.
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al.. Faculty Opinions recommendation of Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2005; 7 (10): 082–089. DOI: 10.3410/f.1029077.343528.
- Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, et al.. The development of the bladder trigone, the center of the anti-reflux mechanism. Development 2007; 134 (20): 3763–3769. DOI: 10.1242/dev.011270.
- Tanaka ST, Ishii K, Demarco RT, Pope JC, Brock JW, Hayward SW. Endodermal Origin of Bladder Trigone Inferred From Mesenchymal-Epithelial Interaction. J Urol 2010; 183 (1): 386–391. DOI: 10.1016/j.juro.2009.08.107.
- Mackie GG, Stephens FD. Duplex Kidneys: A Correlation of Renal Dysplasia with Position of the Ureteral Orifice. J Urol 1975; 114 (2): 274–280. DOI: 10.1016/s0022-5347(17)67007-1.
- Wen JG, Frøkiaer J, Zhao JB, Ringgaard S, Jørgensen TM, Djurhuus JC. Severe partial ureteric obstruction in newborn rats can produce renal dysplasia. BJU Int 1981; 89 (7): 740–745. DOI: 10.1046/j.1464-410x.2002.02747.x.
- Parada Villavicencio C, Adam SZ, Nikolaidis P, Yaghmai V, Miller FH. Imaging of the Urachus: Anomalies, Complications, and Mimics. Radiographics 2016; 36 (7): 2049–2063. DOI: 10.1148/rg.2016160062.
- Krishnan A, Souza A de, Konijeti R, Baskin LS. The Anatomy and Embryology of Posterior Urethral Valves. J Urol 2006; 175 (4): 1214–1220. DOI: 10.1016/s0022-5347(05)00642-7.
- Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, et al.. A comparison of prostatic development in xenografts of human fetal prostate and human female fetal proximal urethra grown in dihydrotestosterone-treated hosts. Differentiation 2018; 115 (24-45): 37–52. DOI: 10.1016/j.diff.2020.06.001.
- Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction 2001; 21 (2): 187–195. DOI: 10.1530/rep.0.1210187.
- LASNITZKI ILSE, MIZUNO TAKEO. Prostatic Induction: Interaction Of Epithelium And Mesenchyme From Normal Wild-type Mice And Androgen-insensitive Mice With Testicular Feminization. J Endocrinol 1980; 85 (3): 423–np. DOI: 10.1677/joe.0.0850423.
- Meeks JJ, Schaeffer EM. Genetic Regulation of Prostate Development. J Androl 2011; 32 (3): 210–217. DOI: 10.2164/jandrol.110.011577.
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol 2003; 253 (2): 165–174. DOI: 10.1016/s0012-1606(02)00031-3.
- Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: Theory of endodermal differentiation. Differentiation 1999; 64 (2): 115–122. DOI: 10.1046/j.1432-0436.1999.6420115.x.
- Hadidi AT, Roessler J, Coerdt W. Development of the human male urethra: A histochemical study on human embryos. J Pediatr Surg 2014; 49 (7): 1146–1152. DOI: 10.1016/j.jpedsurg.2014.01.009.
- Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation 2012; 84 (3): 261–268. DOI: 10.1016/j.diff.2012.06.003.
- Satoh M. Histogenesis and organogenesis of the gonads in human embryos. Med Electron Microsc 1991; 27 (3-4): 254–256. DOI: 10.1007/bf02349668.
- McKay DG, Hertig AT, Adams EC, Danziger S. Histochemical observations on the germ cells of human embryos. Anat Rec 1953; 117 (2): 201–219. DOI: 10.1002/ar.1091170206.
- Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortés L, et al.. SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech Dev 2000; 91 (1-2): 403–407. DOI: 10.1016/s0925-4773(99)00307-x.
- Yao HH-C. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol 2005; 230 (1-2): 87–93. DOI: 10.1016/j.mce.2004.11.003.
- Berta P, Hawkins JB, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, et al.. Genetic evidence equating SRY and the testis-determining factor. Nature 1990; 348 (6300): 448–450. DOI: 10.1038/348448a0.
- Rey RA, Grinspon RP. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract Res Clin Endocrinol Metab 2011; 25 (2): 221–238. DOI: 10.1016/j.beem.2010.08.013.
- McElreavey K, Barbaux S, Ion A, Fellous M. The genetic basis of murine and human sex determination: a review. Heredity (Edinb) 1995; 75 (6): 599–611. DOI: 10.1038/hdy.1995.179.
- Vilain E, McCabe ERB. Mammalian Sex Determination: From Gonads to Brain. Mol Genet Metab 1998; 65 (2): 74–84. DOI: 10.1006/mgme.1998.2749.
- Sinisi AA, Pasquali D, Notaro A, Bellastella A. Sexual Differentiation. Encyclopedic Dictionary of Genetics, Genomics and Proteomics 2003; 26 (3 Suppl): 473–494. DOI: 10.1002/0471684228.egp11478.
- Biason-Lauber A, Chaboissier M-C. Ovarian development and disease: The known and the unexpected. Semin Cell Dev Biol 2015; 45 (59-67): 59–67. DOI: 10.1016/j.semcdb.2015.10.021.
- Rey R, Lukas-Croisier C, Lasala C, Bedecarrás P. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 2003; 211 (1-2): 21–31. DOI: 10.1016/j.mce.2003.09.007.
- Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J Obstet Gynaecol Res 2010; 36 (5): 929–937. DOI: 10.1111/j.1447-0756.2010.01272.x.
- Barteczko KJ, Jacob MI, Jacob MI. Development, Shape and Fate of Gubernaculum Hunteri and Processus Vaginalis Peritonei - Own Phases of Testicular Descent. Adv Anat Embryol Cell Biol 2000; 156:iii-x: 17–72. DOI: 10.1007/978-3-642-58353-7_4.
- Hawkins JR, Taylor A, Berta P, Levilliers J, Auwera B Van der, Goodfellow PN. Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal. Hum Genet 1992; 88 (4): 471–474. DOI: 10.1007/bf00215684.
- Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Mol Cell Endocrinol 2012; 354 (1-2): 34–53. DOI: 10.1016/j.mce.2011.09.035.
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003; 4 (12): 969–980. DOI: 10.1038/nrg1225.
- Cunha GR. The dual origin of vaginal epithelium. Am J Anat 1975; 143 (3): 387–392. DOI: 10.1002/aja.1001430309.
- Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. J Pediatr 1975; 86 (3): 395–398. DOI: 10.1016/s0022-3476(75)80969-3.
- Zalel Y, Pinhas-Hamiel O, Lipitz S, Mashiach S, Achiron R. The development of the fetal penis-anin uterosonographic evaluation. Ultrasound Obstet Gynecol 2001; 17 (2): 129–131. DOI: 10.1046/j.1469-0705.2001.00216.x.
- Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, et al.. The Role of Sonic Hedgehog-Gli2 Pathway in the Masculinization of External Genitalia. Endocrinology 2011; 152 (7): 2894–2903. DOI: 10.1210/en.2011-0263.
- BASKIN LS, LEE YT, CUNHA GR. Neuroanatomical ontogeny of the human fetal penis. BJU Int 1997; 79 (4): 628–640. DOI: 10.1046/j.1464-410x.1997.00119.x.
- Kaplan GW, Lamm DL. Embryogenesis of Chordee. J Urol 1975; 114 (5): 769–772. DOI: 10.1016/s0022-5347(17)67140-4.
- Baskin L, Erol A, Jegatheesan P, Li Y, Liu W, Cunha G. Urethral seam formation and hypospadias. Cell Tissue Res 2001; 305 (3): 379–387. DOI: 10.1007/s004410000345.
- Kluth D, Fiegel HC, Geyer C, Metzger R. Embryology of the distal urethra and external genitals. Semin Pediatr Surg 2011; 20 (3): 176–187. DOI: 10.1053/j.sempedsurg.2011.03.003.
- Omar HA, Hummel M, Jones EA, Perkins KC. Hypoplastic external genitalia in association with X;autosome chromosome translocation. J Pediatr Adolesc Gynecol 1999; 12 (3): 161–164. DOI: 10.1016/s1038-3188(99)00011-x.
- Ferguson-Smith MA. Karyotype-phenotype Correlations in Gonadal Dysgenesis and Their Bearing on the Pathogenesis of Malformations. J Med Genet 1965; 2 (2): 142–155. DOI: 10.1136/jmg.2.2.142.
Ultima atualização: 2024-02-16 21:59