38: Cloacal Anomaly
Este capítulo levará aproximadamente 25 minutos para ler.
Introduction
Anorectal anomalies comprise a spectrum of congenital malformations involving the rectum and anus. Children with anorectal malformations (ARM) are often diagnosed with “imperforate anus” because there is no opening where the normal anal opening should be. This oversimplifies the underlying pathology which frequently involves the urogenital system, spine, and pelvic floor musculature. At one end of the spectrum are minor anomalies in which the anal canal is present, but the anus is anteriorly displaced or covered by perineal skin. With more severe malformations, the rectum fails to reach the perineum and instead connects to the genitourinary tract.
The most severe manifestation of anorectal malformation in girls is a cloacal malformation in which the rectum, urethra, and vagina join to form a single confluent channel which opens onto the perineum. Classically, the perineal opening in a cloaca will be anterior on the perineum, at the site where the urethra would normally open. Posterior cloacal anomalies are a rare variant in which the opening is posteriorly deviated, with the urogenital sinus inserting into or just anterior to an orthotopic rectum.1,2
Embryology and Epidemiology
Cloacal malformation occurs in approximately 1 in 25,000–50,000 live births. The embryological cause is incompletely understood, but involves failed division of the primitive cloaca by the urorectal septum and Rathke’s folds during early gestation.3 Anomalous cloacal septation is associated with abnormal development of other organ systems that form in close temporal and spatial proximity including vertebrae, ureters, kidneys, and Müllerian duct derivatives. When one child is born with an ARM, there is a 1% risk that future siblings will have an ARM.4
In cloacal malformation, the vagina, rectum, and urethra are combined into a single common channel. Hydrocolpos can develop when the vagina fills with urine and mucus, and the distended vaginal cavity can cause urinary tract obstruction via external compression of the ureters or bladder outlet. Patients with a long common channel (> 3 cm) are more likely to have sacral dysplasia and additional congenital anomalies, poor rectal and urinary sphincter musculature, and are more likely to require more complex surgical reconstruction including vaginal reconstruction.
Pathogenesis and Associated Anomalies
Urologic anomalies in patients with anorectal malformations are common and are broadly underappreciated. They are particularly prevalent in girls with cloacal malformation. Pediatricians, pediatric surgeons, and urologists must be aware of the high incidence of urologic comorbidity in these patients, and all patients should be screened for genitourinary anomalies. Reported rates of associated genitourinary anomalies in ARM patients vary widely from 18 to 85%.5 This variability can largely be attributed to differences in completeness of screening. Most series with active screening protocols report a prevalence of around 50% across all ARM types, with increasing rates of urologic anomalies in more severe ARM subtypes.6 Cloacal malformations are the most severe subtype of female anorectal malformation, and the vast majority of these patients will have genitourinary comorbidities (Table 1). This underscores the importance of including urologists as core member of the multidisciplinary team needed to care for patients with anorectal malformations.5,7
Table 1 Prevalence of urologic anomalies in 712 children with anorectal malformations treated at the Nationwide Children’s Hospital, Columbus, Ohio. The prevalence of most reported urologic anomalies increases with increasing severity of the ARM. In brackets, prevalence ranges are reported ranging from minor ARM (perineal) to severe ARM (long-channel cloaca in female, bladder neck fistula in male). Anomalies without differences across ARM type are denoted with an asterisk, and no range is reported. Lower urinary tract dysfunction and Müllerian anomalies were not included in this analysis. Adapted from Fuchs et al 2021.6
| Urologic anomaly | Cloaca < 3 cm (n=55) |
Cloaca > 3 cm (n=60) |
|---|---|---|
| >1 Urologic diagnosis | 72.7% | 93.9% |
| >2 Urologic diagnosis | 36.4% | 71.7% |
| Hydronephrosis | 47.3% | 76.7% |
| Vesicoureteral reflux | 21.8% | 31.8% |
| Solitary kidney | 12.7% | 25.0% |
| Renal fusion anomaly | 7.3% | 11.7% |
| Duplex kidney | 5.5% | 8.3% |
Upper Urinary Tract Anomalies
Coexisting anatomical anomalies of the upper and lower urinary tract occur in 50–60% of patients with anorectal malformations. Commonly seen anomalies include hydronephrosis, vesicoureteral reflux, renal dysplasia, agenesis, duplication, and ectopic variants including simple renal ectopia, cross-fused ectopia, horseshoe kidneys, and ureteral ectopia. The pattern of upper urinary tract anomalies (along with male genital anomalies) from one recent robust single series study is summarized in Table 1.6
In addition to structural anomalies, it has been reported nearly 50% of girls with cloacal anomalies develop severe renal impairment. Consequently, early detection of renal impairment is critical, as is proactive monitoring and management of the lower urinary tract.8
Lower Urinary Tract Dysfunction
Bladder dysfunction is common among all patients with ARM. This is usually associated with a coexisting spinal anomaly, however some children will have innate bladder dysfunction with a normal bony sacrum and spinal cord.9,10 Particularly in cloacal malformation, acquired bladder dysfunction may result from neuromuscular damage during surgical repair of the anorectal malformation.11,12 While meticulous surgical technique and minimization of monopolar electrocautery is prudent, some neuromuscular damage may be unavoidable due to the more midline location of pelvic autonomic plexus in patients with ARM.13 While screening with uroflowmetry is likely adequate for patients with minor ARM and normal spinal imaging, we advocate all girls with cloacal anomalies undergo urodynamic evaluation.
Genital Anomalies
Anomalies of the female genital tract are less readily apparent on external examination, yet Müllerian abnormalities are very common among girls with ARM.14 Cloacal malformations are associated with a very high rates of anomalous internal genitalia, with reports ranging from 53 to 67%.15 Some degree of Müllerian duplication is commonly seen, ranging from vaginal septae to complete uterine and vaginal duplication. Müllerian structures may also be underdeveloped, and asymmetry in duplicated reproductive tracts in common. Delineating the Müllerian anatomy in girls with ARM is critical. Hydrocolpos is common and can lead to pain, obstructive uropathy, infection, and infertility. Clinicians must assess and treat hydrocolpos in newborns, in whom the vaginal fluid is primarily urine collecting in the reproductive tract, and again in pubertal patients after menarche, in whom menstrual products can collect in the vagina(s). Rates of menstrual obstruction are reported around 40%.8 In these patients, vaginal obstruction can be congenital or due to vaginal stricture, which is common following posterior sagittal anorectovaginourethroplasty (PSARVUP).14,16
Spinal Anomalies
Approximately one quarter to one third of patient with ARM will have associated vertebral or spinal cord pathology.17,18,19 Among patients with cloacal malformation specifically, small series suggest much higher rates of spinal anomalies (over 70%), though a recent large retrospective review reports 42% of these patients have spinal pathology.20,21 Common anomalies include spinal cord tethering, sacral hypoplasia, hemisacrum (which is associated with a presacral mass. Spinal pathologies can lead to urologic, neurologic, and orthopedic complications.17 Routine spinal imaging is standard of care, and children with spinal pathologies need to be monitored for neurogenic bladder.
VACTERL Association
Multiple syndromes and genetic disorders have been described which include anorectal malformations as a feature. The most significant of these is the VACTERL association, though many others have been described. VACTERL is an acronym for vertebral anomalies, anorectal anomalies, cardiac anomalies, tracheoesophageal fistula, renal anomalies, and limb anomalies. Most cases are sporadic rather than hereditary. Not all components of the VACTERL association are expressed in every patient. The most common are vertebral, anorectal, and renal abnormalities. To diagnose VACTERL/VACTER Syndrome, at least 3 of defects must be present.22 Other syndromes that may be commonly associated with ARMs include MURCS (Müllerian duct aplasia, renal aplasia, and cervicothoracic somite dysplasia), OEIS (omphalocele, exstrophy, imperforate anus, and spinal defects).23
Evaluation and Diagnosis
Prenatal
Cloacal malformation can be diagnosed prenatally due to the increased incidence of concurrent anomalies and hydrocolpos. The advantage of prenatal diagnosis is two-fold; parents can be counseled, and arrangements can be made to deliver at a center familiar with neonatal management of anorectal malformations. Clues on prenatal ultrasound include dilated bowel, calcified meconium, lack of meconium in the rectum, hydrocolpos (usually identified as a pelvic cystic structure and a poorly visualized bladder), renal anomalies, neural tube defects, and absent radius, among others. Abnormalities of multiple systems may strengthen suspicion for VACTERL or another syndrome with anorectal anomalies. Some centers have pursued prenatal MRI in hopes of confirming the diagnosis, though this too is imperfect and is not the current standard of care.24,25
Physical Examination
While prenatal diagnosis of cloacal malformation can be suspected on prenatal imaging, confirmation of the diagnosis is based on physical exam postnatally. When approaching the perineal and genital exam, urologists should evaluate the number and location of perineal openings as well as the gluteal cleft. In girls, there should be three separate perineal openings (urethra, vagina, anus), with the anal orifice centered within the anal sphincter complex and separated from the vagina by the perineal body. A single perineal orifice in a girl is pathognomonic for a cloaca and is typically found in the expected location of the urethra. There can be wide variations of the appearance of the perineum, but the single orifice is the critical finding on exam (Figure 1). A less common variant of cloacal malformation, called a posterior cloacal variant has a perineal opening more posterior where the vagina or anus would be located (Figure 2). Occasionally, prominent preputial skin will give the appearance clitoromegaly. In the absence of palpably enlarged corporal bodies, this should not prompt routine endocrinologic evaluation for intersex disorders.26
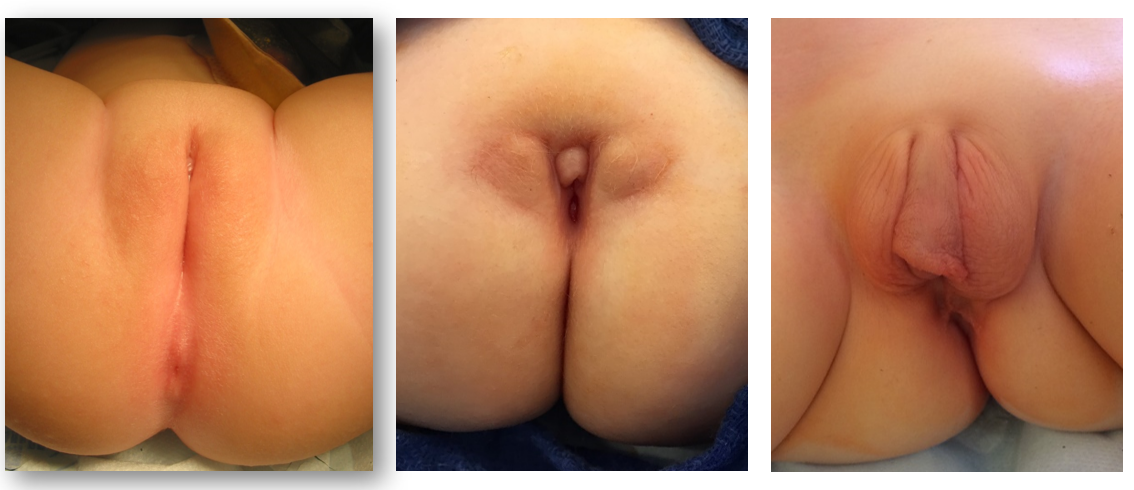
Figure 1 Perineal photographs of girls with cloacal malformation. The anal opening is absent, and the single perineal opening located posterior to the clitoris, near the expected location of the urethra. Redundant preputial tissue is common.
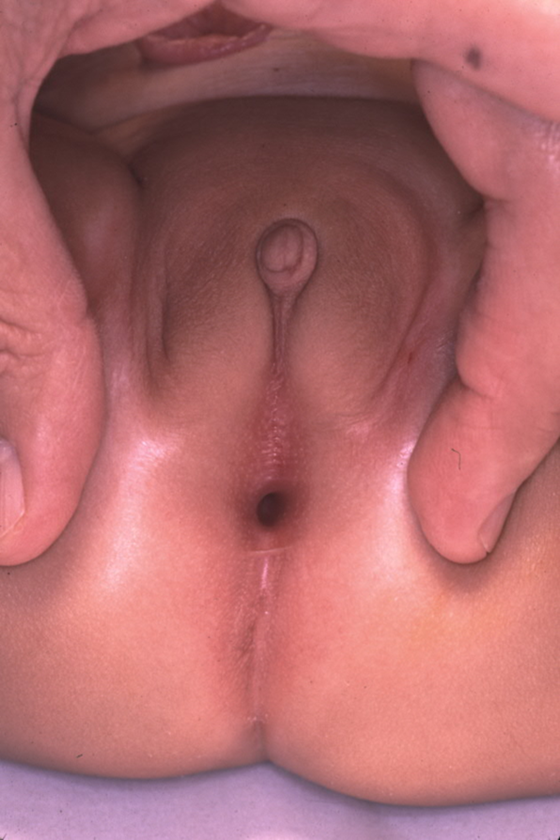
Figure 2 Posterior cloacal anomaly, with the single perineal opening posteriorly displaced toward the expected location of the anus.
Labs and Imaging
Subsequent workup focuses on evaluating potential associated anomalies.
Echocardiogram
This is needed to evaluate structural cardiac anomalies, particularly prior to anesthesia.
Complete Abdominal Ultrasound
Assess for renal anomalies, urinary tract obstruction, hydrocolpos, and Müllerian duplication.
Spinal Imaging
A spinal ultrasound should be obtained, primarily to assess for tethered cord. Plan radiography is useful for assessing the sacrum following ossification of the coccyx. Anterior-posterior and lateral sacral x-rays may demonstrate sacral anomalies such as a hemisacrum and sacral hemivertebrae and allow for assessment of the degree of sacral hypoplasia. In general, more severe hypoplasia portends poorer continence outcomes.27 Measurement of the “sacral ratio” has been proposed as a prognostic indicator for bowel continence, but has not proven reliable as a predictor of urinary continence, development of neurogenic bladder, or need for intermittent catheterization.28
For patients with spinal anomalies, neurosurgical evaluation and spinal MRI should follow, and these patients need ongoing urologic monitoring for neurogenic bladder. Children who present after 3–4 months of age will need a spinal MRI, as ossification of the vertebral arches obscures the sonographic window.
Renal Function Testing
Obtain serum creatinine to assess baseline renal function. Note that renal function assessment in children is imperfect, and renal immaturity in neonates may underestimate renal function, particularly in premature babies. Cystatin C is increasingly being used as a corollary to serum creatinine.
Additional imaging is commonly pursued as clinically indicated. In the setting of hydronephrosis due to hydrocolpos or bladder distension, a repeat renal ultrasound after hydrocolpos drainage should be obtained to confirm improvement of hydronephrosis. Renal scintigraphy or MR urography may be pursued if there is concern for poor renal drainage or to establish relative renal function. VCUG can screen for vesicoureteral reflux, though the role of routine preoperative voiding cystourethrogram is debatable and it may be difficult to blindly catheterize the bladder. Lastly, although there is a high incidence of abnormal urodynamic findings, urodynamics need not be pursued until after surgical reconstruction.
Preoperative Planning
The remainder of the workup is spent defining the anatomy in preparation for surgical reconstruction.
Colostogram
A high-pressure distal colostogram will define the level of the rectal fistula, evaluate the length of distal colon available for pull-through, and define the relationship of the rectum to the sacrum and coccyx. Following colostomy creation, a Foley catheter is inserted into the mucus fistula and the balloon is gently inflated to prevent leakage. Contrast is then instilled through the catheter to opacify the distal colon. The pressure of the instilled contrast must be adequate to overcome the tone of the levator muscles, and contrast must be water-soluble and isosmotic due to the rare risk of colon perforation. Alternatively, “invertogram” may be used to investigate the extent of the defect in anal or rectal atresia. The anus is marked with radioopaque marker, the baby is inverted such that air in the rectum rises to the highest point, and a lateral radiograph is taken. The distance between the air and the radioopaque marker indicates the distance from the rectum to the perineal skin.
Endoscopy
Cystoscopy and vaginoscopy is essential to the preoperative planning for repair of a cloaca or urogenital sinus. This may be coordinated with diverting colostomy or another procedure to avoid unnecessary anesthesia. Endoscopy will define the length of the common channel and distance from the bladder neck to the confluence, characterize the bladder neck and sphincter complex, and assess the bladder and ureters. Absence of orthotopic ureteral orifices should raise suspicion for an ectopic ureter. Vaginoscopy will reveal the presence of a vaginal septum, vaginal duplication, and size of the vagina(s) for future reconstruction.
It is particularly important to establish the location of the vaginal confluence relative to the bladder neck.29 This distance represents the urethral length, and has implications for surgical repair and continence outcomes.30 Shorter uretheral length indicates a more severe malformation.29
Genitography
Fluoroscopic genitograms are commonly used to delineate the urologic, gynecologic, and rectal structures. A Foley catheter is inserted into the perineal opening and contrast is instilled. Conventional two-dimensional fluoroscopic studies are subject to overlap of opacified structures and are thus limited in their ability to define three-dimensional relationships and obtain accurate measurements, highlighting the need for endoscopic evaluation. Many centers now employ three-dimensional computed tomography or magnetic resonance imaging to delineate anatomy in complex malformations (Figure 3).31,32
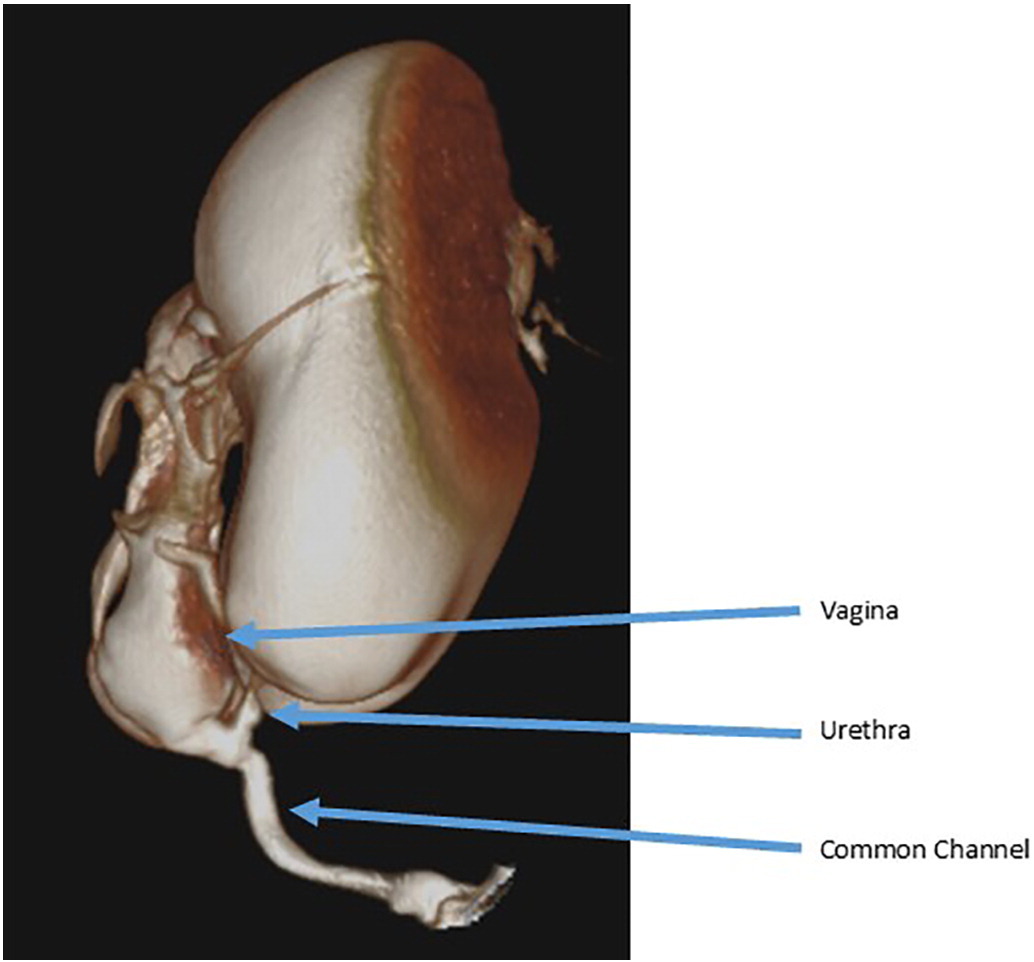
Figure 3 Example of a three-dimensional cloacogram showing a long common channel with a high vaginal confluence and a short urethra.
A three-dimensional cloacogram (or standard pelvic magnetic resonance imaging) is also useful for delineating Müllerian anatomy and ureteral ectopy. Urologists should be aware of a condition known as obstructed hemivagina and ipsilateral renal anomaly (OHVIRA), in which one hemivagina is obstructed thus vaginoscopy will demonstrate a single vagina and cervix. Suggestion of Müllerian anomalies on ultrasound that are discordant with endoscopy should be further investigated, and the internal genitalia should be directly inspected when there is opportunity such as at the time of initial diverting colostomy.16
Treatment Options, Outcomes, and Complications
Newborn Period
It is critical in the newborn period to ensure adequate resuscitation of the infant and decompression of the obstructed organ systems. Initial management must include a colostomy and mucous fistula in the neonatal period, typically within the first 24–48 hrs of life. This allows for decompression of the colon and separation of the fecal stream from the urinary tract.
Hydrocolpos Management
Hydrocolpos and a distended bladder with fluid and it is common in girls with cloacal malformation and develops as urine refluxes up the vagina as a result of distal urinary obstruction. The vaginal dilation can be severe enough to cause upper tract urinary obstruction and urine can reflux out the fallopian tubes causing urinary ascites. There is no consensus on management of hydrocolpos in the newborn period, but it does require drainage if there is obstructive uropathy. Traditionally drainage has been achieved with surgical decompression by means of a vaginostomy, percutaneous vaginostomy tube, or vesicostomy. More recently, the less invasive option of clean intermittent catheterization of the common channel has been utilized.33 When the common channel is catheterized, the catheter typically will preferentially enter the vagina rather than the bladder. In a comparison of the two methods of decompression, surgical and CIC, there was no difference in creatinine trend providing good evidence that CIC is a good, non-invasive, initial option for decompression of hydrocolpos.33 If a vaginal septum is present and vaginostomy may only drain one side of the vagina and inadequately decompress the system. In this case, a vaginostomy on the other side of the vagina or incision of the vaginal septum can allow for better drainage.
An important principle of all methods of hydrocolpos decompression is follow up to confirm successful drainage. After starting CIC for hydrocolpos, ultrasound should confirm decompression of the vagina and resolution of urinary tract dilation. If it is unsuccessful, a more invasive option should be considered. If hydronephrosis persists despite decompression of the vagina, workup for other causes of hydroureteronephrosis should be completed and neurogenic bladder should be suspected.
Bladder Management
Many girls with cloacal malformation will not need any intervention for bladder emptying. If a hostile or high-pressure bladder with incomplete emptying is identified, a cutaneous vesicostomy can be created to allow for adequate decompression of the urinary tract. Clean intermittent catheterization is typically not possible because, as mentioned above, the catheter preferentially enters the vagina because the urethral takeoff is commonly anterior. Vesicostomy may also be indicated in girls with high grade vesicoureteral reflux or recurrent infections. Again, drainage and decompression of the urinary tract needs to be confirmed with ultrasound.
Surgical Repair of Cloacal Malformation
Timing of Repair
With the obstructed organ systems decompressed, primary surgical repair of the cloacal malformation does not need to occur immediately. This delay allows for adequate bonding period between the family and the child but also provides time to assemble the multi-disciplinary team involved in the child’s care. This period also allows for optimized nutrition and strength. There is no consensus on the optimal age of repair but repair < 6 months after birth has been shown to have greater rate of post-operative complications, specifically wound dehiscence and most agree that 6–12 months of age seems to be the ideal age for primary repair.34
Surgical Techniques
Surgical approach is dictated by the anatomy on cloacogram and cystovaginoscopy. There are two surgical techniques used for the repair: total urogenital mobilization and urogenital separation. The principles of surgical repair are to mobilize the urethra, vagina and rectum down to the perineum so there are three separate orifices. This is done through a posterior sagittal incision and may be performed in combination with a laparotomy if needed. The posterior sagittal approach with the baby in the prone position is the initial approach. This positioning allows for improved visualization of the anatomy compared to lithotomy. First, the rectum is identified entering the common channel and mobilized superiorly to the peritoneal reflection. At this point in the operation, a total urogenital mobilization or a urogenital separation is performed.
Urogenital Separation
Urogenital separation was the initial technique described for repair of cloacal malformation and was the standard approach popularized by Hendren (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10).35 It remains the approach of choice for girls with a common channel > 3 cm in length. In the urogenital separation, the rectum, vagina, and urethra are separated, and individually brought to the perineum. After the rectum is mobilized off the common channel, the vagina is then carefully dissected free from the posterior wall of the urethra, bladder neck and bladder. The defect in the urethra where the vagina was mobilized away is then closed primarily over a catheter. This dissection can be challenging, and care should be taken not to devascularize or denervate the urinary tract. The vagina should be mobilized superiorly as much as possible to allow for it to reach the perineal skin without tension. If the vagina does not reach, an abdominal approach may be needed to maximize mobilization. Once the vagina reaches the skin, the vagina is brought down to the perineal skin, secured posterior to the urethra and then around laterally to create the neo-introitus. The perineal body is then recreated, and the rectum placed within the muscle complex. If the vagina still does not reach after mobilization, there are additional vaginoplasty techniques that have been described such as vaginal switch technique, a bowel interposition graft or even bringing the native vagina down on tension with the expectation that it may scar down and require introitoplasty or buccal vaginoplasty at the onset of puberty. The decision about vaginoplasty technique is a multi-disciplinary discussion between gynecology, colorectal and urologic surgery and should involve discussion pre-operatively with the family.
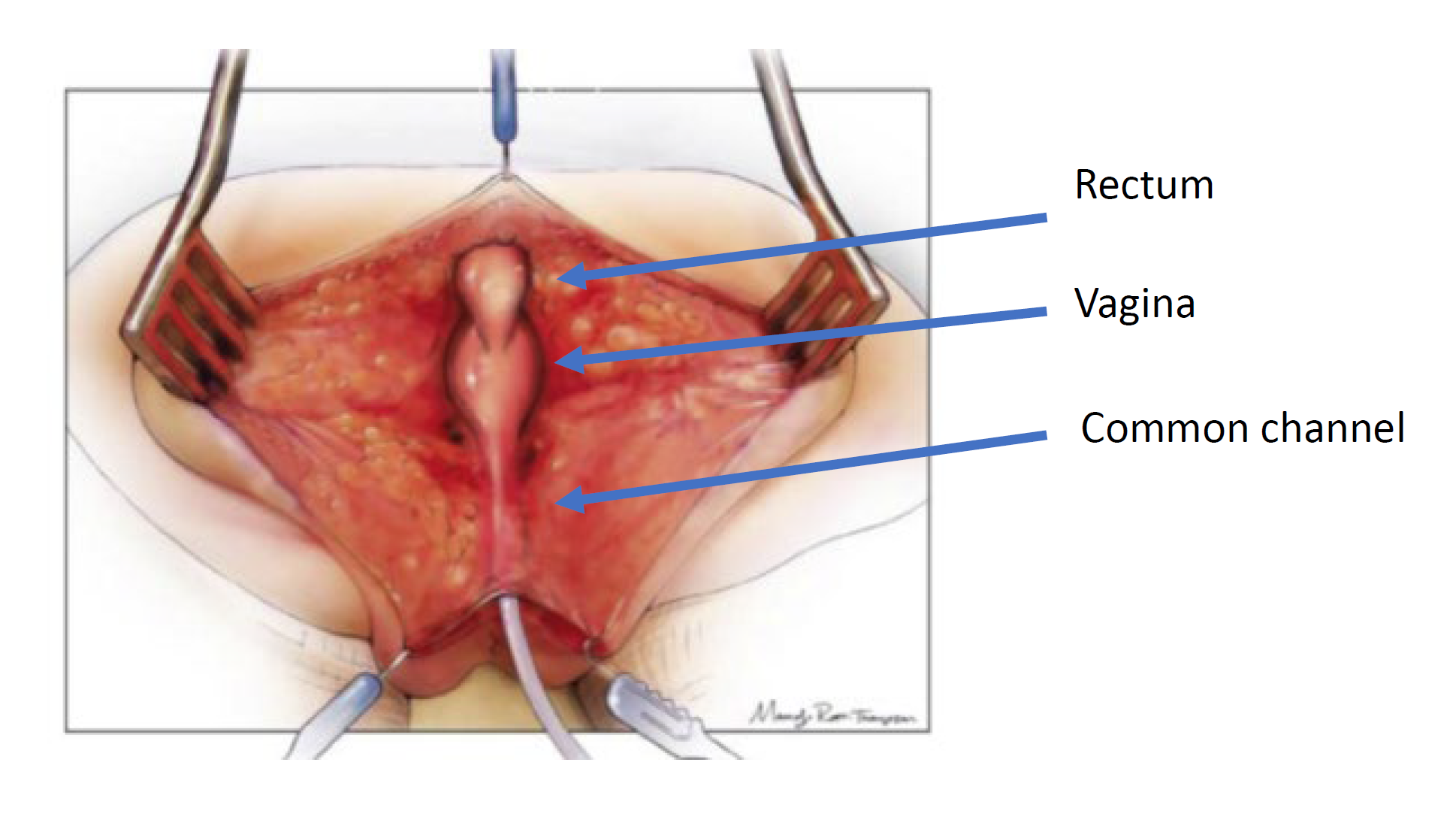
Figure 4 Illustration of a urogenital separation. The patient is in the prone position, and a posterior sagittal incision has been made, exposing the posterior surfaces of the rectum, vagina, and common channel. (continued to Figure 5)
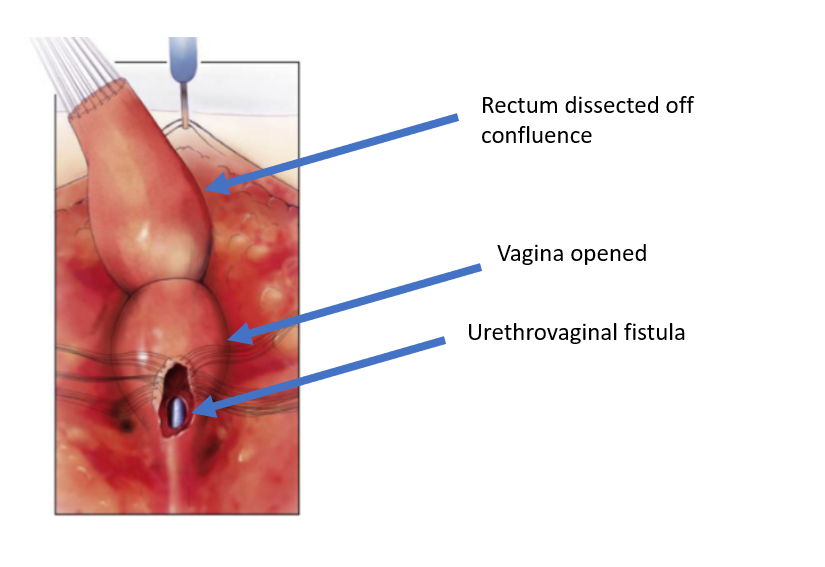
Figure 5 The rectum has been dissected off the vaginal confluence, leaving an openin in the vagina. (continued from Figure 4, continued to Figure 6)
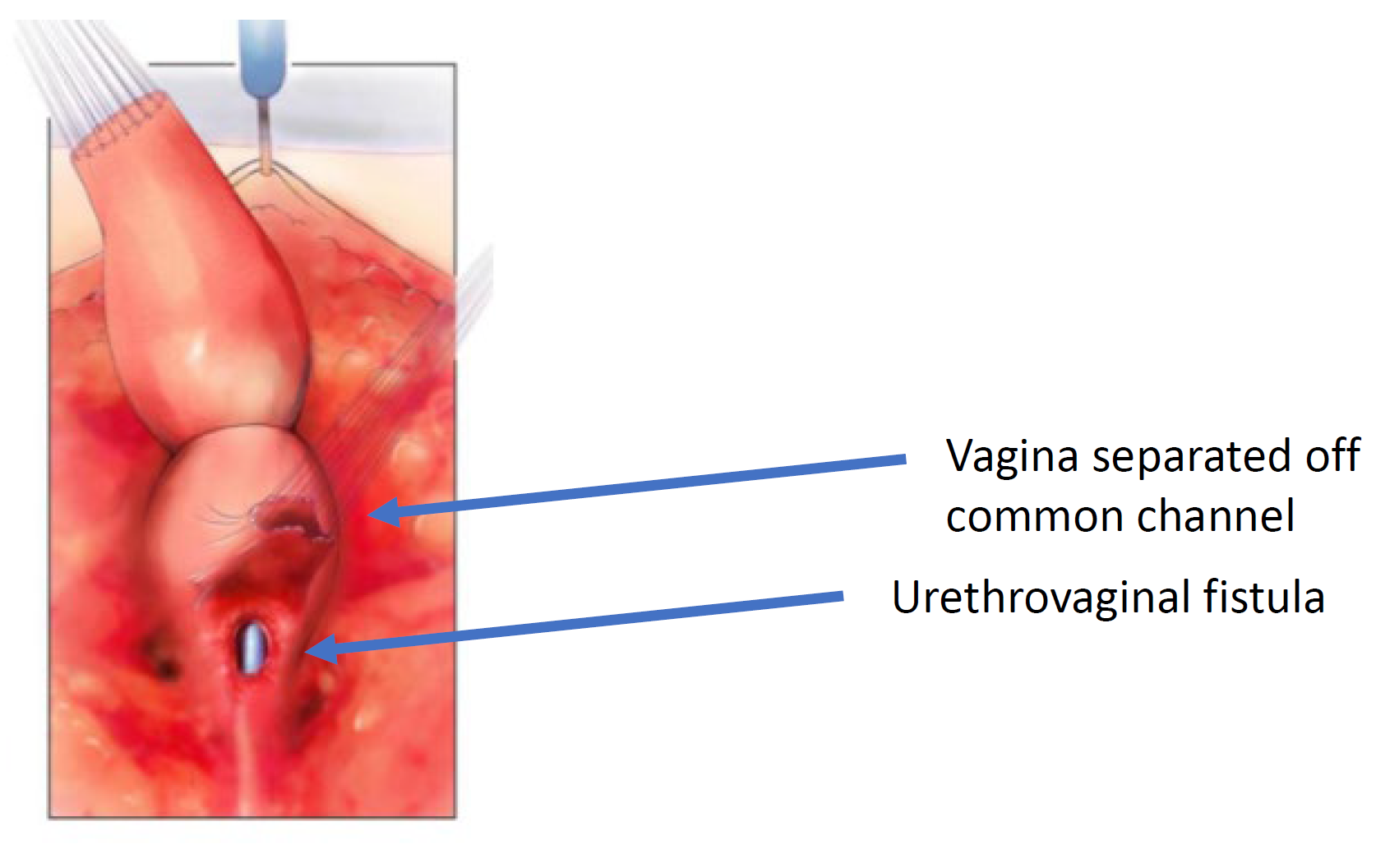
Figure 6 The vagina has been dissected off the common channel at its confluence with the urethra. The opening through which the catheter is seen is the urethrovaginal fistula. (continued from Figure 5, continued to Figure 7)
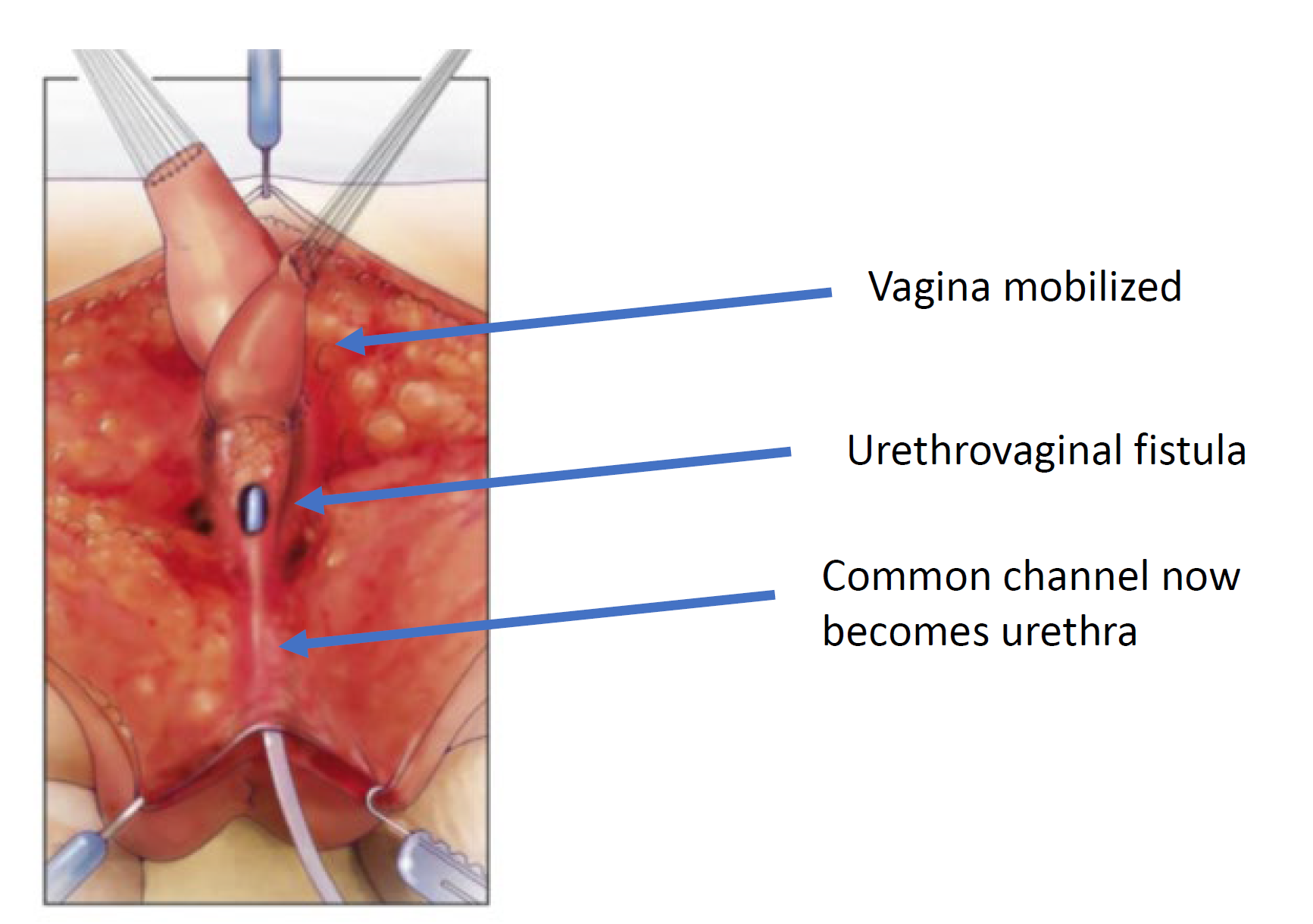
Figure 7 The vagina is mobilized proximally to gain length and elasticity such that it can reach the perineum. (continued from Figure 6, continued to Figure 8)
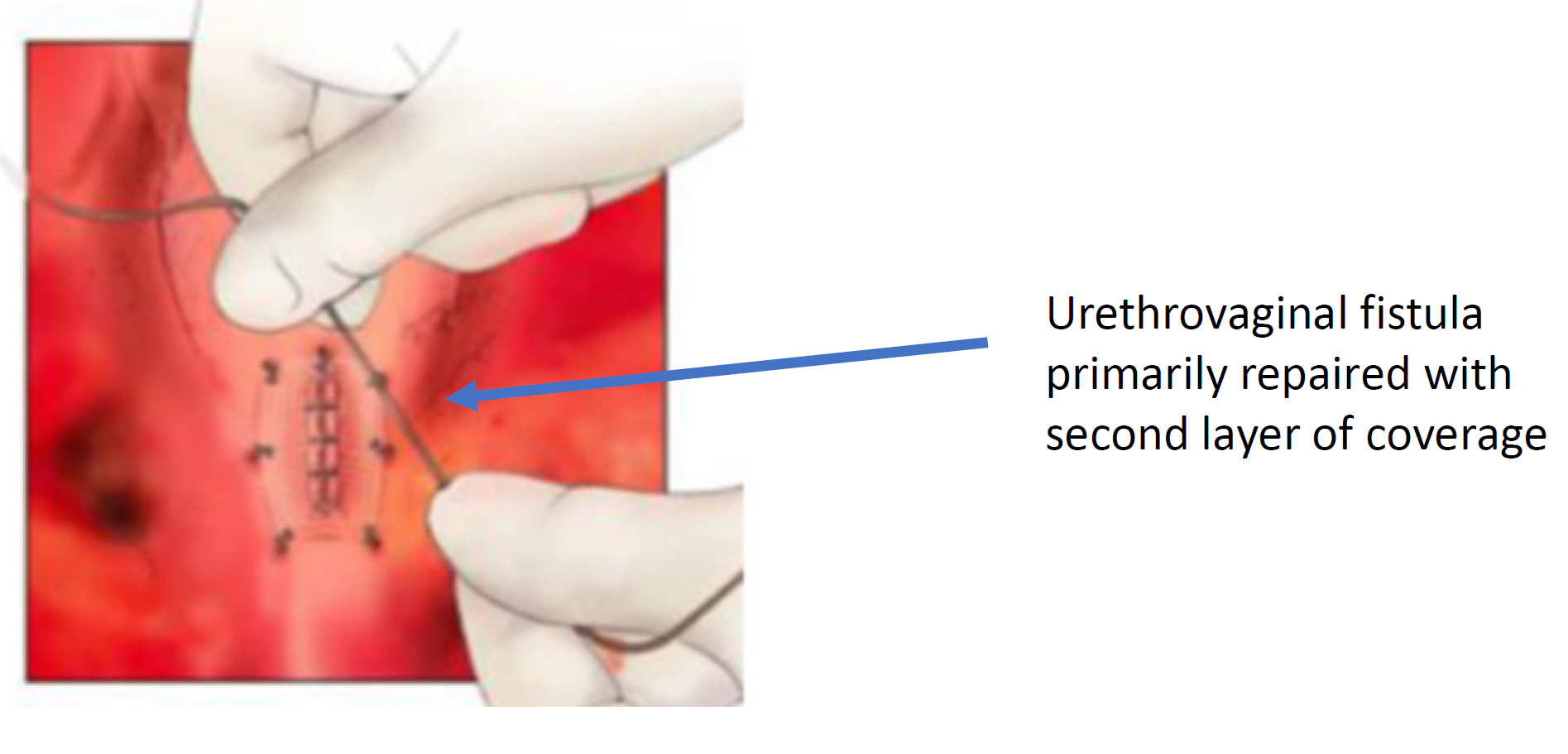
Figure 8 The urethrovaginal fistula is closed. (continued from Figure 7)
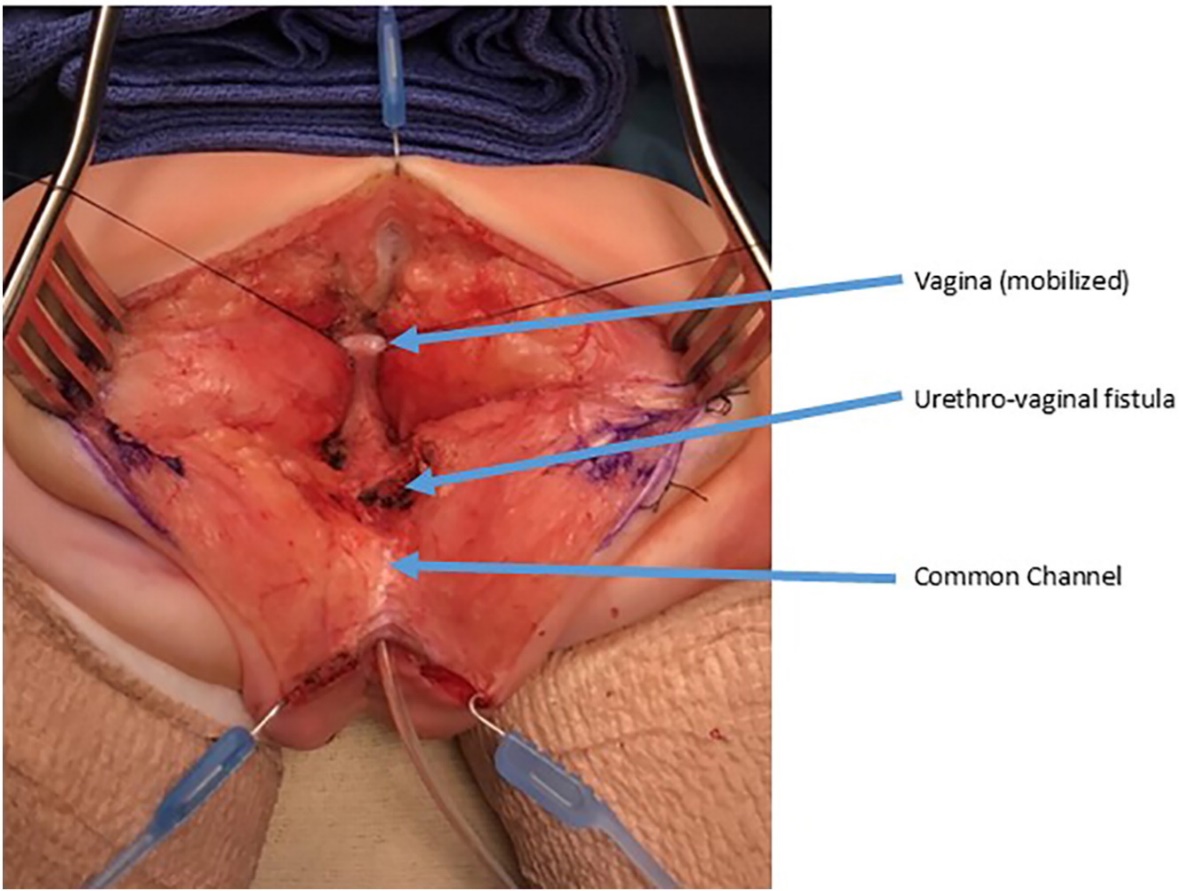
Figure 9 Intraoperative photographs of a urogenital separation. A posterior sagittal incision has been made. A catheter is in the common channel. The vagina is held with traction sutures. The confluence of the vagina and urethra is labels as urethrovaginal fistula, which will be closed after separating the vagina from the urethra. (continued to Figure 10)
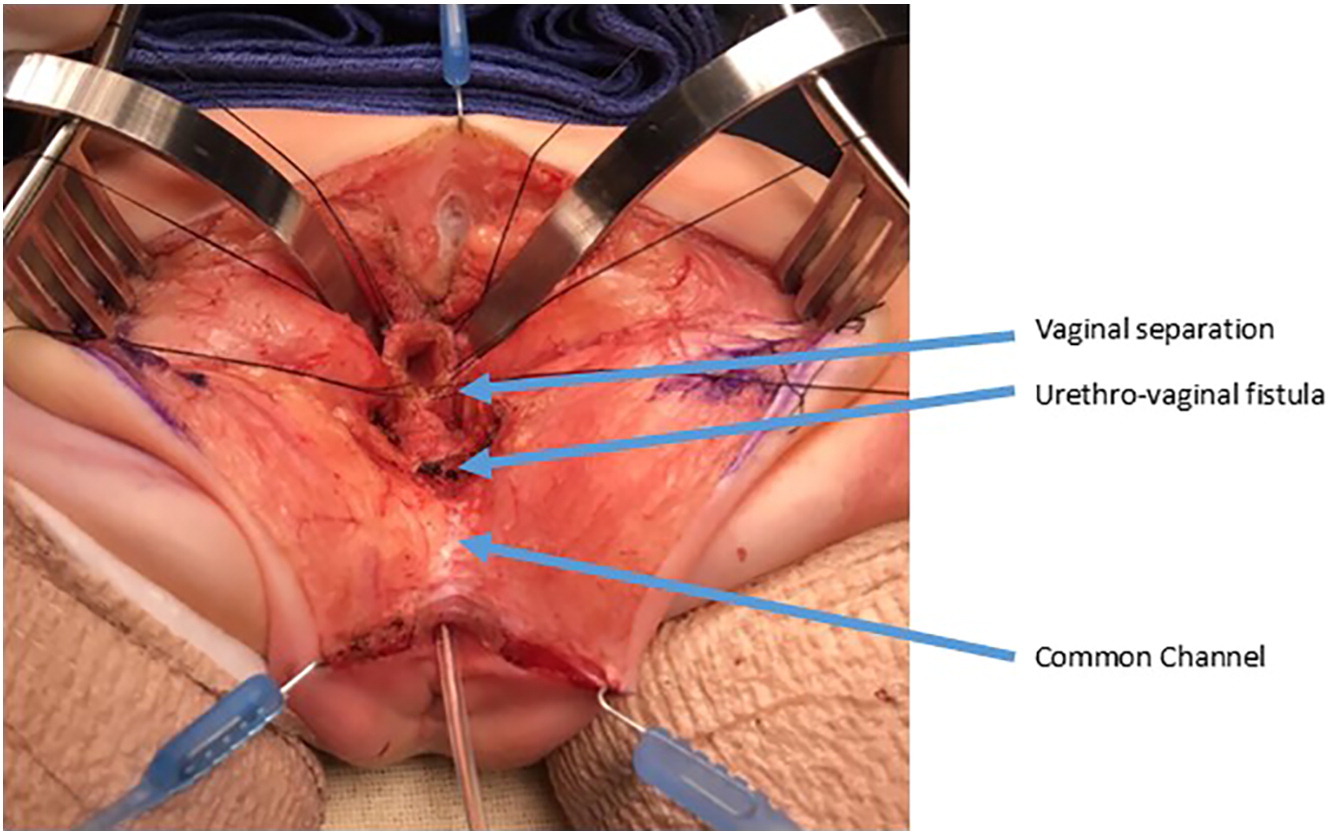
Figure 10 Intraoperative photographs of a urogenital separation. The vagina as been opened and separated from the urethra. The vagina will then be mobilized proximally to gain length to reach the perineum. The urethrovaginal fistula is again labeled and will be closed in layers primarily over a catheter. (continued from Figure 9)
Total Urogenital Mobilization
A total urogenital mobilization was first introduced for cloacal repair by Peña in 1997 and was considered an “easier approach to cloaca” because it does not require separating the vagina from the urinary tract (Figure 11, Figure 12, Figure 13).36 Rather, this is a pull-through technique in which the urogenital sinus is mobilized until the confluence reaches the perineum, where the urethra and vagina are separated and anastomosed to the perineum. Peña proposed that this technique would reduce urethrovaginal fistula and devascularization of the urinary tract. Because the urinary tract cannot be mobilized as extensively as the vagina, there are limitations to how far the common channel can be mobilized with this technique, it is best utilized in short common channel (< 3 cm) cloacal malformation. Once the rectum is mobilized off the common channel, the common channel is circumferentially dissected from the perineum and the common channel opened up until the confluence of the urethra and the vagina reaches the perineal skin without tension. The common channel is split open and used for a labioplasty. The urethra anastomosed to the anterior edge of the introitus, just posterior to the clitoral tissue. The urethra and vaginal introitus is anastomosed to the skin without tension. The perineal body is recreated and the rectum brought to the skin within the sphincter muscle complex.
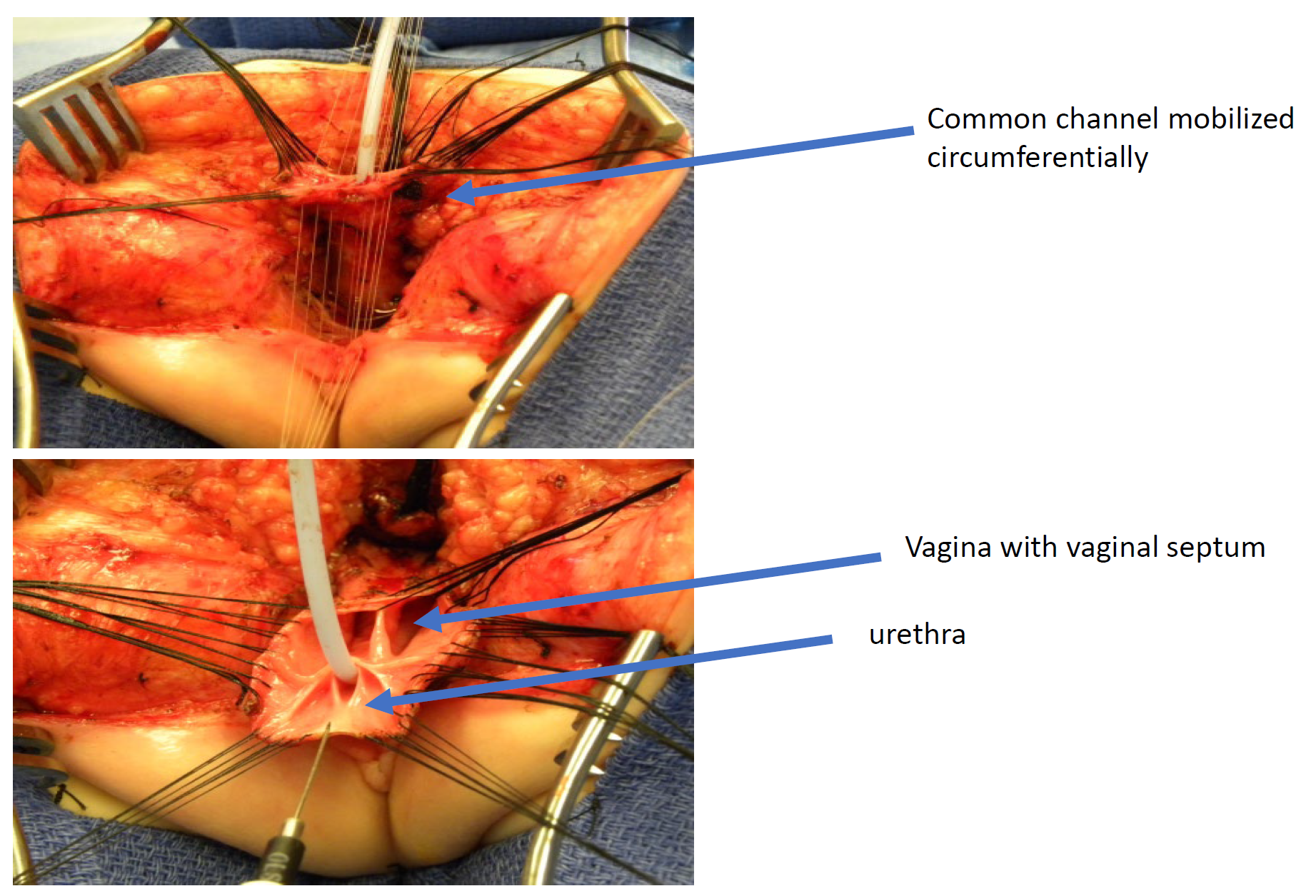
Figure 11 Intraoperative photographs of a total urogenital mobilization (TUM) cloacal repair. A posterior sagittal incision has been made, and the common channel and vaginas have been mobilized together toward the perineum. In this photograph, the rectum has already been separated.
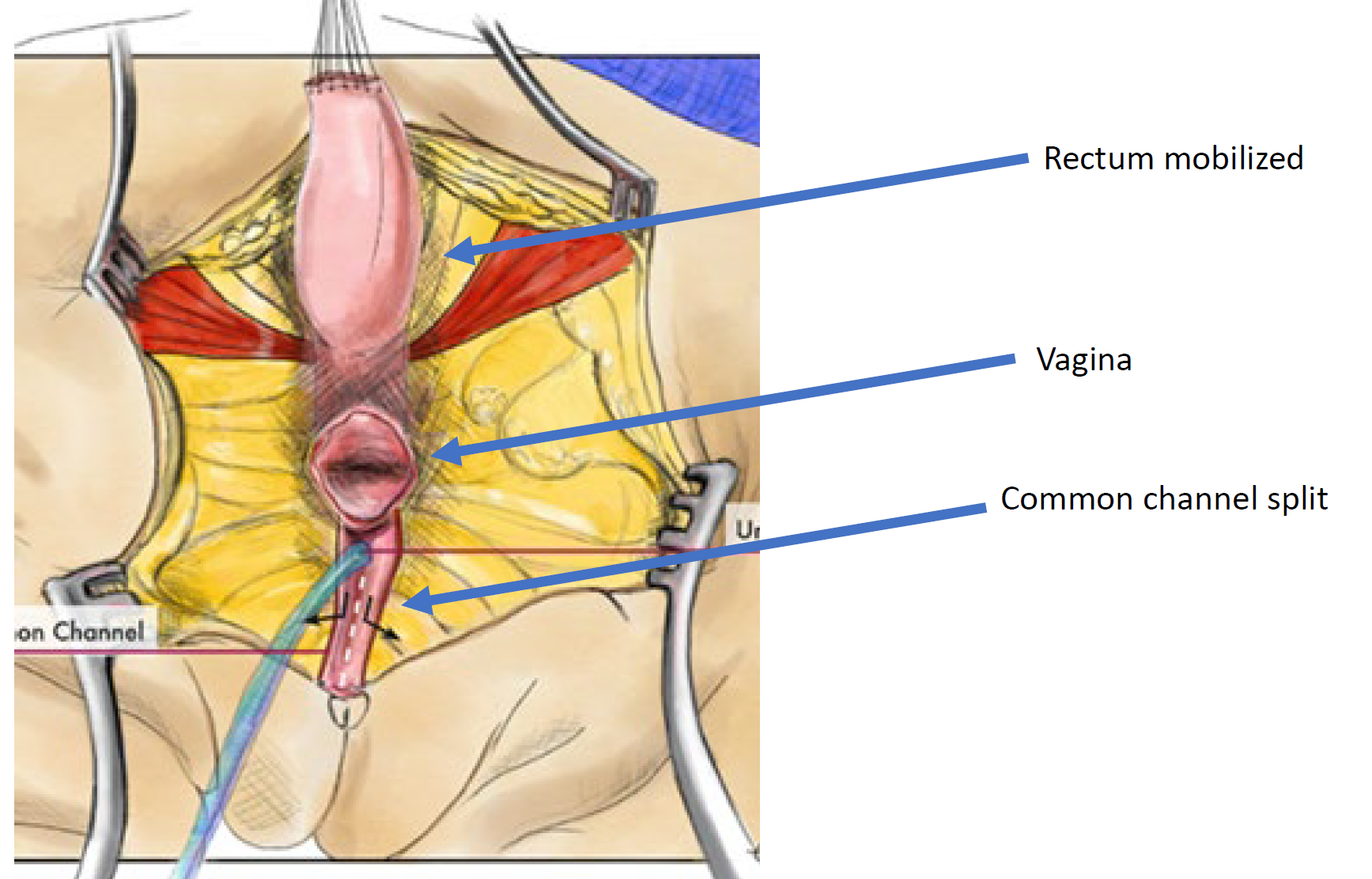
Figure 12 Illustration of a total urogenital mobilization (TUM) cloacal repair. The mobilization is complete once the confluence reaches the perineum and the urethra, vagina, and rectum can be separately anastomosed to the perineum. The common channel is split, and redundant tissue is rotated to line the introital opening. (continued to Figure 13)
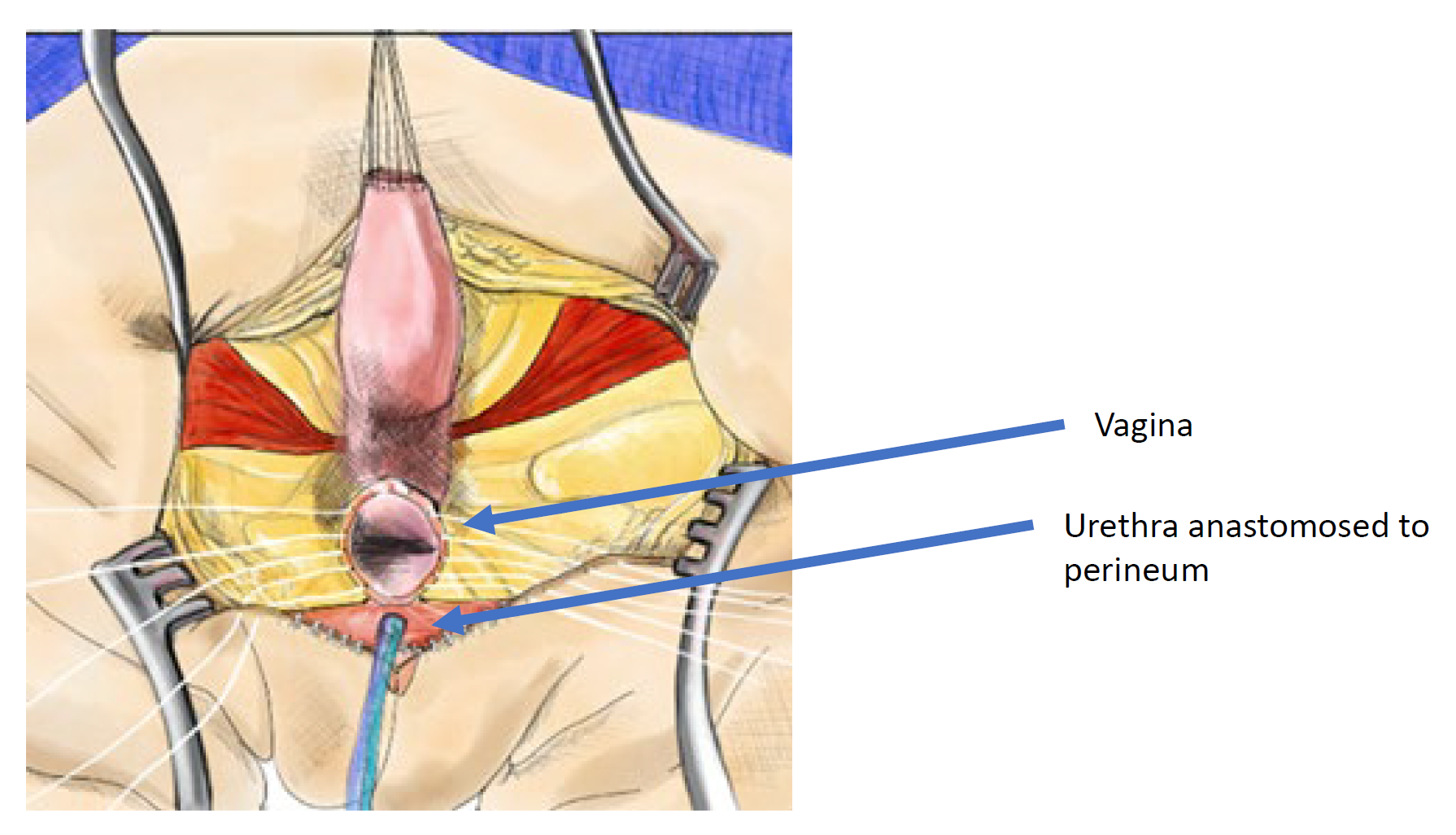
Figure 13 Illustration of a total urogenital mobilization (TUM) cloacal repair. The mobilization is complete once the confluence reaches the perineum and the urethra, vagina, and rectum can be separately anastomosed to the perineum. The common channel is split, and redundant tissue is rotated to line the introital opening. (continued from Figure 12)
Determining Surgical Technique
Historical Perspective
The urogenital separation was the primary technique for cloacal repair and often required a posterior sagittal incision along with a laparotomy. This technique is technically demanding and time consuming. Early reports of a high-volume surgeon reported reasonable results reporting 58.8% voiding spontaneously, 28.4% who perform CIC and 2.8% who have urinary diversion.35 This study reports “urinary control” but no details about urinary accidents, emptying or renal function are reported. Peña’s description of the total urogenital mobilization introduced a simpler approach to cloacal malformation. After a large number of TUM procedures, he reported shorter operative times, hospitalization and improved urinary continence (74%) and voluntary bowel movements.37 However, he did report that in those with long common channels (> 3 cm) cloaca, there was a high complexity of intra-operative decision making and often, after the common channel was completely mobilized and it would not reach, the vagina was then separated off the mobilized common channel leaving the urethra and bladder neck circumferentially mobilized with compromised vascularity and likely denervation. This puts the urethra at risk of devascularization and necrosis.
These two important experiences in the late 1990s resulted in a surgical algorithm based on measurement of the common channel. Short common channel (< 3 cm) would be best served with a total urogenital mobilization, while a long common channel (> 3 cm) would be recommended to have a urogenital separation with or without laparotomy or laparoscopy and bowel interposition graft if the vagina did not reach the skin. This algorithm relies greatly upon accurate measurement of the common channel. Measurement can be done endoscopically under visualization, or on 2-dimensional or 3-dimensional reconstructed cloacogram (Figure 14). However, 3-dimentional reconstructed cloacogram has been shown to be the most accurate measurement of the common channel and allows less experienced surgeons and trainees comprehend this complex anatomy at the same level as senior surgeons enhancing preparedness for the surgical procedure.38,39
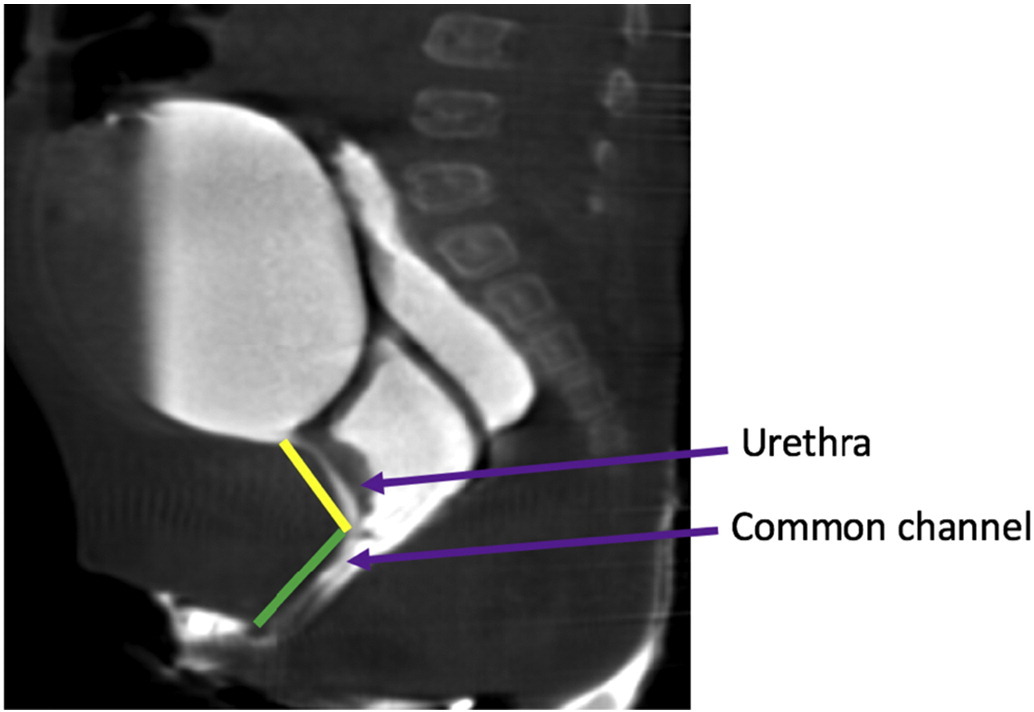
Figure 14 Sagittal T2 MRI images of the cloacal malformation delineating the urethra and the common channel lengths.
Development of Current Surgical Algorithm
For nearly 2 decades, surgical decision making based solely on common channel length was the standard of care. However, as multi-disciplinary care became more prevalent and urologists were more closely involved in the care of these patients along with pediatric surgery, it was observed that while most girls with short common channels had longer urethras, there is an unusual subtype of girls with short common channels who have very short urethras. In this subset of patients, if a TUM were to be performed, it would result in a very short urethra and the bladder neck being positioned right at the perineum and very poor continence potential. As a result of this observation an updated algorithm was developed incorporating both common channel length and urethral length in determining the surgical approach Figure 15.13 In this algorithm if a common channel is > 3 cm, regardless of urethral length, a UG separation is performed. If a common channel is < 3 cm and the urethra is > 1.5 cm, then a TUM is the operative approach of choice. The unusual variant that has a common channel < 3 cm and a urethra < 1.5 cm, this new algorithm would dictate that the child should have a UG separation, thereby maintaining the anatomic position of the bladder and bladder neck within the pelvis and avoiding mobilizing it down and under the pelvic diaphragm. This surgical algorithm was the first time in over 20 years since Peña’s description of the TUM that a new surgical technique had been proposed and it has been well accepted across high volume centers. Results of this algorithm have been excellent with regards to reduced complexity of decision making and change of plan intra-operatively. With accurate pre-operative assessment of urethral and common channel measurements, significant urologic comorbidity can be avoided and results in a patent and catheterizable urethra in 97% of patients.40
Figure 15 Suggested surgical algorithm for cloacal malformation.13
Post Operative Management
Wound management and catheter drainage is paramount after posterior sagittal cloacal repair. There is no consensus about the length of time for urethral catheterization after cloacal repair but this typically ranges from 1–2 weeks in TUM and up to 4 weeks in UG separation. Some advocate for vesicostomy drainage of the bladder but this is not required. The posterior sagittal and introital incisions are at risk of dehiscence so avoiding any straddling or splits is critical during healing. It is also advised that perineal pressure is avoided, and children not be held by their perineum or be sitting in jumpers or straddle toys. Postoperative anatomy is commonly evaluated with a thorough clinic exam or cystoscopy and exam under anesthesia in the operating room.
Bladder Management Post-Operatively
After surgical repair of the cloacal malformation, it is important to ensure adequate bladder emptying. Surgical mobilization and underlying bladder dysfunction can lead to urinary retention or incomplete emptying. This can be managed by having all children perform intermittent catheterization after catheter removal and adjust schedule of catheterization based on urine volumes. Others use pre-operative urodynamic assessment to aid in decision making about CIC. There is no consensus about post-operative bladder management with catheterization. In a recent report of 18 girls who underwent cloacal repair, 44.4% required assisted bladder emptying (CIC or vesicostomy) while the remainder were diapered.41 Factors associated with need for CIC after repair were long common channel length, UG separation and need for assisted bladder emptying prior to surgical repair. Importantly, diaper management pre-operatively did not guarantee that assisted bladder emptying could be avoided post-operatively.
Vaginal Management Post-Operatively
Post-operative patency of the vaginal introitus is commonly assessed with a thorough office exam or exam under anesthesia. If the vaginal introitus becomes stenotic early in childhood, this can be observed until she is older and begins puberty. If the stenosis is severe enough to prevent outflow of menses, this should be managed prior to menarche. Ultrasound surveillance of the reproductive tract is recommended beginning 6–9 months after breast bud development and continuing every 6–9 months through menarche, assessing endometrial thickness in each uterine body.15 If obstructed menstrual flow is detected, gynecology or endocrinology should be consulted to initiate hormonal suppression to temporize until surgical intervention. Preferred operative techniques for introital or vaginal stenosis include simple introitoplasty or buccal mucosal vaginoplasty.42 Many centers have pediatric and adolescent gynecology specialists who monitor for this, though urologists may assume this care where pediatric gynecologists are unavailable.
Long-Term Outcomes
Bladder Function
Urinary continence is challenging to assess due to the complexity and heterogeneity of the population. Hendren reported ~60% spontaneous voiding with “urinary control” and Peña similarly reported 54% continence and 46% requiring clean intermittent catheterization. However, neither of these studies report definitions of continence or report rates of bladder reconstruction.35,36 More recently, there was a review of long-term outcomes for 50 patients older than 3 years with repaired cloacal malformation. This group reports 80% of girls achieving “social continence”, of whom 22% void spontaneously while the remainder perform CIC.43 This same group had 46% who required a mean number of 4.7 surgical procedures to achieve continence. Again, definition of continence is not well defined and many who are “socially continent” had reconstructive surgery. There is a great need for long-term follow up in this population to determine factors associated with achieving continence and reliable continence rates.
Renal Function
Renal insufficiency is a constant concern in girls with cloacal malformation. The largest series of 64 patients reports greater than 50% have GFR < 80 mL/min/1.73 m2 with 17% having end stage renal disease, 6% requiring transplant and 6% deceased from renal failure.44 This same group stresses that, renal impairment appears to be acquired over time as 66% of girls who progressed to chronic renal insufficiency had normal serum nadir creatinine. Other smaller series report far more favorable results ranging from 66–100% of girls with normal renal function at 5–9 year follow up.45,46,47 Interestingly, these series are more recent and are all institutions with multi-disciplinary teams caring for these girls. Again, long-term follow up from high volume centers evaluating renal function in these patients is greatly needed.
Key Points
- Cloacal malformation represents the most severe female anorectal malformation
- Urologic and systemic comorbidities are common including upper tract anomalies, and Müllerian anomalies, and bladder dysfunction. Comprehensive urologic evaluation multidisciplinary care is paramount.
- Initial management prioritizes early drainage of hydrocolpos and diverting colostomy
- Surgery to repair cloacal malformation is calls a posterior sagittal anorectovaginorectoplasty (PSARVUP). There are two primary surgical approaches: total urogenital mobilization (for less severe malformations) and urogenital separation (for more severe anomalies)
- Many girls with require bladder catheterization. Long term data on continence outcomes and renal function is lacking
Suggested Readings
- Levitt MA, Peña A. Pitfalls in the management of newborn cloacas. Pediatr Surg Int 2005; 21: 264–269. DOI: 10.1007/s00383-005-1380-2.
- Harris KT, Kong L, Vargas M, Hou V, Pyrzanowski JL, Desanto K, et al.. Considerations and outcomes for adolescents and young adults with cloacal anomalies: a scoping review of urologic, colorectal, gynecologic and psychosocial concerns. Urology 2023. DOI: 10.1016/j.urology.2023.08.047.
References
- AbouZeid AA, Mohammad SA, Shokry SS, El-Naggar O. Posterior cloaca: A urogenital rather than anorectal anomaly. J Pediatr Urol 2021; 17 (3): 410.e1–410.e7. DOI: 10.1016/j.jpurol.2021.01.014.
- Peña A, Bischoff A, Breech L, Louden E, Levitt MA. Posterior cloaca–further experience and guidelines for the treatment of an unusual anorectal malformation. J Pediatr Surg 2010; 45 (6): 1234–1240. DOI: 10.1016/j.jpedsurg.2010.02.095.
- Thomas DFM. The embryology of persistent cloaca and urogenital sinus malformations. Asian J Androl 2019; 22 (2): 124. DOI: 10.4103/aja.aja_72_19.
- Falcone RA, Levitt MA, Peña A, Bates M. Increased heritability of certain types of anorectal malformations. J Pediatr Surg 2007; 42 (1): 124–128. DOI: 10.1016/j.jpedsurg.2006.09.012.
- Skerritt C, DaJusta DG, Fuchs ME, Pohl H, Gomez-Lobo V, Hewitt G. Long-term urologic and gynecologic follow-up and the importance of collaboration for patients with anorectal malformations. Semin Pediatr Surg 2020; 29 (6): 150987. DOI: 10.1016/j.sempedsurg.2020.150987.
- Fuchs ME, Halleran DR, Bourgeois T, Sebastião Y, Weaver L, Farrell N, et al.. Correlation of anorectal malformation complexity and associated urologic abnormalities. J Pediatr Surg 2021; 56 (11): 1988–1992. DOI: 10.1016/j.jpedsurg.2021.02.051.
- Vilanova-Sanchez A, Halleran DR, Reck-Burneo CA. Re: A Descriptive Model for a Multidisciplinary Unit for Colorectal and Pelvic Malformations. J Urol 2019; 203 (4): 651–651. DOI: 10.1097/ju.0000000000000723.04.
- Warne SA, Wilcox DT, Creighton S, Ransley PG. Long-Term Gynecological Outcome of Patients with Persistent Cloaca. J Urol 2003; 170 (4 Part 2): 1493–1496. DOI: 10.1097/01.ju.0000086702.87930.c2.
- Mosiello GIOVANNI, Capitanucci MARIALUISA, Gatti CLAUDIA, Adorisio OTTAVIO, Lucchetti MARIACHIARA, Silveri MASSIMILIANO, et al.. How to Investigate Neurovesical Dysfunction in Children With Anorectal Malformations. J Urol 2003; 170 (4 Part 2): 1610–1613. DOI: 10.1097/01.ju.0000083883.16836.91.
- Goossens WJH, Blaauw I de, Wijnen MH, Gier RPE de, Kortmann B, Feitz WFJ. Urological anomalies in anorectal malformations in The Netherlands: effects of screening all patients on long-term outcome. Pediatr Surg Int 2011; 27 (10): 1091–1097. DOI: 10.1007/s00383-011-2959-4.
- Jindal B, Grover V, Bhatnagar V. The Assessment of Lower Urinary Tract Function in Children with Anorectal Malformations before and after PSARP. Eur J Pediatr Surg 2009; 19 (01): 34–37. DOI: 10.1055/s-2008-1039027.
- Warne STEPHANIEA, Godley MARGARETL, Wilcox DUNCANT. Surgical Reconstruction Of Cloacal Malformation Can Alter Bladder Function: A Comparative Study With Anorectal Anomalies. J Urol 2004; 172 (6 Part 1): 2377–2381. DOI: 10.1097/01.ju.0000145201.94571.67.
- Davies MRQ. Anatomy of the nerve supply of the rectum, bladder, and internal genitalia in anorectal dysgenesis in the male. J Pediatr Surg 1997; 32 (4): 536–541. DOI: 10.1016/s0022-3468(97)90702-8.
- Cardamone S, Creighton S. A gynaecologic perspective on cloacal malformations. Curr Opin Obstet Gynecol 2015; 27 (5): 345–352. DOI: 10.1097/gco.0000000000000205.
- Breech L. Gynecologic concerns in patients with anorectal malformations. Semin Pediatr Surg 2010; 19 (2): 139–145. DOI: 10.1053/j.sempedsurg.2009.11.019.
- Breech L. Gynecologic concerns in patients with cloacal anomaly. Semin Pediatr Surg 2016; 25 (2): 90–95. DOI: 10.1053/j.sempedsurg.2015.11.006.
- Levitt MA, Patel M, Rodriguez G, Gaylin DS, Pena A. Tethered Spinal Cord in Patients with Anorectal Malformations. Anorectal Malformations in Children 1997; 2 (3): 281–286. DOI: 10.1007/978-3-540-31751-7_18.
- Kim SM, Chang HK, Lee MJ. Re: Spinal Dysraphism With Anorectal Malformation: Lumbosacral Magnetic Resonance Imaging Evaluation of 120 Patients. J Urol 2010; 185 (3): 1094–1094. DOI: 10.1016/s0022-5347(11)60163-8.
- Beaufort CMC de, Groenveld JC, Mackay TM, Slot KM, Beer SA de, Jong JR de, et al.. Spinal cord anomalies in children with anorectal malformations: a retrospective cohort study. Pediatr Surg Int 2023; 39 (1): 281–286. DOI: 10.1007/s00383-023-05440-y.
- Muller CO, Crétolle C, Blanc T, Alova I, Jais J-P, Lortat-Jacob S, et al.. Impact of spinal dysraphism on urinary and faecal prognosis in 25 cases of cloacal malformation. J Pediatr Urol 2014; 10 (6): 1199–1205. DOI: 10.1016/j.jpurol.2014.05.012.
- Garvey EM, Fuller M, Frischer J, Calkins CM, Rentea RM, Ralls M, et al.. Multi-Institutional Review From the Pediatric Colorectal and Pelvic Learning Consortium of Minor Spinal Cord Dysraphism in the Setting of Anorectal Malformations: Diagnosis, Treatment, and Outcomes. J Pediatr Surg 2023; 58 (8): 1582–1587. DOI: 10.1016/j.jpedsurg.2023.04.009.
- Inserm. VACTERL/VATER association. Definitions 2011; 6 (56). DOI: 10.32388/m8pjt5.
- Levitt MA, Peña A. Anorectal malformations. Pediatr Surg Int 2007; 3-3 (2-3). DOI: 10.1007/bf00182758.
- Jacobs SE, Tiusaba L, Al-Shamaileh T, Bokova E, Russell TL, Ho CP, et al.. Fetal and Newborn Management of Cloacal Malformations. Children (Basel) 2022; 9 (6): 888. DOI: 10.3390/children9060888.
- Dannull KA, Browne LP, Meyers MZ. The spectrum of cloacal malformations: how to differentiate each entity prenatally with fetal MRI. Pediatr Radiol 2019; 49 (3): 387–398. DOI: 10.1007/s00247-018-4302-x.
- Bischoff A, Trecartin A, Alaniz V, Hecht S, Wilcox DT, Peña A. A cloacal anomaly is not a disorder of sex development. Pediatr Surg Int 2019; 35 (9): 985–987. DOI: 10.1007/s00383-019-04511-3.
- Vilanova-Sanchez A, Reck CA, Sebastião YV, Fuchs M, Halleran DR, Weaver L, et al.. Can sacral development as a marker for caudal regression help identify associated urologic anomalies in patients with anorectal malformation? J Pediatr Surg 2018; 53 (11): 2178–2182. DOI: 10.1016/j.jpedsurg.2018.03.018.
- VanderBrink BA, Reddy PP. Early urologic considerations in patients with persistent cloaca. Semin Pediatr Surg 2016; 25 (2): 82–89. DOI: 10.1053/j.sempedsurg.2015.11.005.
- Rink RC, Adams MC, Misseri R. A New Classification for Genital Ambiguity and Urogenital Sinus Anomalies. J Urol 2005; 175 (4): 1500–1501. DOI: 10.1016/s0022-5347(05)00847-5.
- Wood RJ, Reck-Burneo CA, Dajusta D. Re: Cloaca Reconstruction: A New Algorithm Which Considers the Role of Urethral Length in Determining Surgical Planning. J Urol 2018; 204 (6): 1368–1368. DOI: 10.1097/ju.0000000000001276.02.
- Patel MN, Racadio JM, Levitt MA, Bischoff A, Racadio JM, Peña A. Complex cloacal malformations: use of rotational fluoroscopy and 3-D reconstruction in diagnosis and surgical planning. Pediatr Radiol 2012; 42 (3): 355–363. DOI: 10.1007/s00247-011-2282-1.
- Halleran DR, Smith CA, Fuller MK, Durhm MM, Dickie B, Avansino JR, et al.. Measure twice and cut once: Comparing endoscopy and 3D cloacagram for the common channel and urethral measurements in patients with cloacal malformations. J Pediatr Surg 2020; 55 (2): 257–260. DOI: 10.1016/j.jpedsurg.2019.10.045.
- Chalmers DJ, Rove KO, Wiedel CA, Tong S, Siparsky GL, Wilcox DT. Clean intermittent catheterization as an initial management strategy provides for adequate preservation of renal function in newborns with persistent cloaca. J Pediatr Urol 2015; 11 (4): 211.e1–211.e4. DOI: 10.1016/j.jpurol.2015.05.014.
- Versteegh HP, Sutcliffe JR, Sloots CEJ, Wijnen RMH, Blaauw I de. Postoperative complications after reconstructive surgery for cloacal malformations: a systematic review. Tech Coloproctol 2015; 19 (4): 201–207. DOI: 10.1007/s10151-015-1265-x.
- Hendren WH. Cloaca, The Most Severe Degree of Imperforate Anus. Ann Surg 1998; 228 (3): 331–346. DOI: 10.1097/00000658-199809000-00006.
- Peña A. Total urogenital mobilization–An easier way to repair cloacas. J Pediatr Surg 1997; 32 (2): 263–268. DOI: 10.1016/s0022-3468(97)90191-3.
- Levitt MA, Peña A. Cloacal malformations: lessons learned from 490 cases. Semin Pediatr Surg 2010; 19 (2): 128–138. DOI: 10.1053/j.sempedsurg.2009.11.012.
- Reck-Burneo CA, Lane V, Bates DG. Re: The Use of Rotational Fluoroscopy and 3-D Reconstruction in the Diagnosis and Surgical Planning for Complex Cloacal Malformations. J Urol 2019; 204 (6): 1367–1368. DOI: 10.1097/ju.0000000000001276.01.
- Gasior AC, Reck C, Lane V, Wood RJ, Patterson J, Strouse R, et al.. Transcending Dimensions: a Comparative Analysis of Cloaca Imaging in Advancing the Surgeon’s Understanding of Complex Anatomy. J Digit Imaging 2019; 32 (5): 761–765. DOI: 10.1007/s10278-018-0139-y.
- Skerritt C, Wood RJ, Jayanthi VR, Levitt MA, Ching CB, DaJusta DG, et al.. Does a standardized operative approach in cloacal reconstruction allow for preservation of a patent urethra? J Pediatr Surg 2021; 56 (12): 2295–2298. DOI: 10.1016/j.jpedsurg.2021.01.011.
- Davis M, Mohan S, Russell T, Feng C, Badillo A, Levitt M, et al.. A prospective cohort study of assisted bladder emptying following primary cloacal repair: The Children’s National experience. J Pediatr Urol 2023; 19 (4): 371.e1–371.e11. DOI: 10.1016/j.jpurol.2023.03.017.
- Leeuwen K van, Baker L, Grimsby G. Autologous buccal mucosa graft for primary and secondary reconstruction of vaginal anomalies. Semin Pediatr Surg 2019; 28 (5): 150843. DOI: 10.1016/j.sempedsurg.2019.150843.
- Warne SA, Wilcox DT, Ransley PG. Long-term Urological Outcome of Patients Presenting with Persistent Cloaca. J Urol 2002; 168(4: 1859–1862. DOI: 10.1097/00005392-200210020-00048.
- Warne SA, Wilcox DT, Ledermann SE, Ransley PG. Renal Outcome in Patients With Cloaca. J Urol 2002; 67 (6): 2548–2551. DOI: 10.1097/00005392-200206000-00055.
- Braga LHP, Lorenzo AJ, Dave S, Del-Valle MH, Khoury AE, Pippi-Salle JL. Long-term renal function and continence status in patients with. Can Urol Assoc J 2007; 1 (4): 371. DOI: 10.5489/cuaj.442.
- DeFoor WR, Bischoff A, Reddy P, VanderBrink B, Minevich E, Schulte M, et al.. Chronic Kidney Disease Stage Progression in Patients Undergoing Repair of Persistent Cloaca. J Urol 2015; 194 (1): 190–194. DOI: 10.1016/j.juro.2015.01.080.
- Rink RC, Herndon CDA, Cain MP, Kaefer M, Dussinger AM, King SJ, et al.. Upper and lower urinary tract outcome after surgical repair of cloacal malformations: a three-decade experience. BJU Int 2005; 96 (1): 131–134. DOI: 10.1111/j.1464-410x.2005.05581.x.
- Levitt MA, Peña A. Pitfalls in the management of newborn cloacas. Pediatr Surg Int 2005; 21: 264–269. DOI: 10.1007/s00383-005-1380-2.
- Harris KT, Kong L, Vargas M, Hou V, Pyrzanowski JL, Desanto K, et al.. Considerations and outcomes for adolescents and young adults with cloacal anomalies: a scoping review of urologic, colorectal, gynecologic and psychosocial concerns. Urology 2023. DOI: 10.1016/j.urology.2023.08.047.
Ultima atualização: 2024-02-16 20:59