31: Distal Hypospadias
Este capítulo levará aproximadamente 20 minutos para ler.
Introduction
In the ancient Greek literature, Aristotle was the first to describe the urinary dysfunction resulting from a penile malformation.1 The Greek physician Galen is credited for coining the term hypospadias in the 100s A.D.1 As its early describers, the etymology of the term hypospadias is Greek, deriving from the roots hypo (under) and spadon (rent, fissure).
Hypospadias is one of the most common congenital anomalies in newborn males. It is characterized by ventral placement of the urethral opening with arrested development of the ventral penis, urethra, and foreskin.2 Due to this arrested development, there is incomplete urethral tubularization, which results in an ectopic urethral opening on the ventral aspect of the penis. Depending on the severity, hypospadias is often associated with ventral chordee (penile curvature) and ventral foreskin deficiency, resulting in a “dorsal hooded” prepuce. The anatomic location of the urethral orifice is typically used for classification of hypospadias. The hypospadic meatus can be located anywhere from the glans to the perineum. About 50% of hypospadias are distal (glans, coronal, and subcoronal), 30% are middle (penile shaft), and 20% are proximal (penoscrotal, scrotal, and perineal) (Figure 1).3,4 This chapter will focus on the embryology, epidemiology, etiology, diagnosis, evaluation, repair, follow-up, and complications of distal hypospadias. Proximal hypospadias is considered a more severe presentation and will be covered in another chapter.
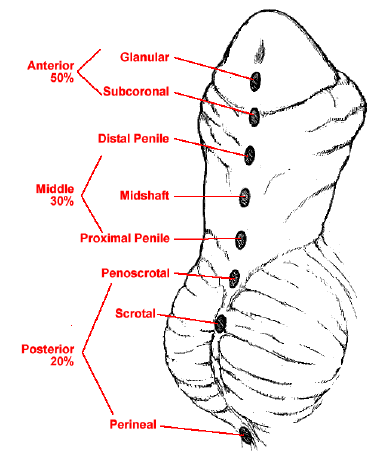
Figure 1 Spectrum of hypospadias presentations.4
Embryology
Development of the external male genitalia begins in an ambisexual stage during the fifth gestational week.5 Until the seventh and eighth weeks, the genital tubercle, urogenital fold, and labioscrotal folds are identical.5,6 Sexual differentiation begins during gestational weeks 8 and 9 when expression of the SRY gene initiates male gonadal differentiation, and thereby the production of androgens.5 However, penile development occurs through both androgen-independent and androgen-dependent events.7,8 Androgen-independent events are common to both males and females and include the formation of the genital tubercle, urethral plate, urethral groove, glans, prepuce, and corporal body.7 Androgen-dependent events include urethral tubularization, circumferential foreskin development, and penile elongation.6,7,8
The male urethra develops through a “double zipper” mechanism beginning in week 8 of gestation.9,10 In this process, the initial opening zipper involves distal canalization of the urethral plate to form the urethral groove. A closing zipper follows in proximal to distal fashion, as the medial edges of the endodermal urethral folds fuse together to form a tubular urethra. The zipper continues to move distally until the urethral meatus is at the glanular tip. This process, along with the circumferential development of foreskin, is driven through androgenic stimulation and is typically complete by week 17 of gestation.7,8
Disruption in this embryologic process causes a failure in proper urethral fold fusion, resulting in a urethral opening on the ventral aspect of the penis.11 Additionally, a disruption prevents the foreskin from developing circumferentially, which presents as a dorsal hooded prepuce in hypospadias.12
Epidemiology
Hypospadias is one of the most common congenital anomalies in males, occurring in about 1:200-300 live births in the US, 1:500 throughout Europe, and 1:2,000-3,000 throughout Asia.5,10,13 Though the incidence of hypospadias varies throughout the world, multiple database studies showed a drastic increase in incidence in recent history. There was a doubling in rate (0.2 to 0.4 percent) in the U.S. during the 1970s and 1980s.14 This increase is unlikely due to improvements in detection, as there was a disproportional increase in severe cases compared to more mild cases. Rather, multifactorial influences such as endocrine disruption, environmental exposures, and genetic predisposition may contribute to this increased rate.11,15 Other population surveys also found drastic increases in the hypospadias incidence during the late 20th century, including studies of Canada, Australia, England, Norway, Denmark, Finland, Japan, and Italy, among others.3,16 A database study from Washington found that advanced maternal age (>40 years old), preexisting maternal diabetes, and white race were each independently associated with increased risk of having a newborn with hypospadias.15
Etiology/Pathogenesis
The pathogenesis of hypospadias is likely due to disruption of androgen metabolism and response during gestation, as androgenic stimulation is responsible for key processes in penile development.6,7,8 These disruptions are likely multifactorial, originating from both genetic predisposition and environmental exposures.17
Genetic
Supporting evidence for a genetic predisposition is through the family clustering reported in hypospadias, as about 7% of affected patients have an additional 1st, 2nd, or 3rd degree family member also affected, especially with the “anterior” or distal type.18 A family history positive for hypospadias was also reported for almost a quarter of boys with hypospadias.19 Paternal history of hypospadias was present in about 31% of affected boys.19
Genes that have been associated with increased risk of hypospadias are related to defects in their androgenic hormonal balance during development. Specifically, genes that affect androgen metabolizing enzymes, androgen receptors, and estrogen receptors.20,21,22,23,24,25,26 For example, the expression of androgen receptors and 5 alpha-reductase type 2 have been localized to ventral urethral epithelium and stroma, respectively, during development.26 Defects in their activity may lead to abnormal urethral remodeling and result in hypospadias.26 Some congenital anomaly syndromes that may also present with hypospadias include WAGR, Denys-Drash, Smith-Lemli-Opitz, Opitz G, and Frasier syndromes, among nearly 200 others.5,18,27
Environmental
There is a two-hit hypothesis regarding the development of hypospadias, in which environmental exposure to endocrine disruptors may potentiate the effects of genetic predisposition to hypospadias.17 Some of the identified exposures that may increase risk of hypospadias include maternal pregnancy-related progestin intake, polybrominated diphenyl ethers (flame retardants), maternal smoking, paternal prescription drug use, maternal diethylstilbestrol exposure, maternal iron supplementation, and paternal exposure to pesticides.28,29,30 Of these, only maternal progestin intake, polybrominated diphenyl ethers, maternal smoking, and paternal prescription drug use have been shown to be statistically significant in studies.
Diagnosis and Evaluation
Hypospadias should be diagnosed at birth during a thorough neonatal physical examination. Performing a neonatal circumcision is contraindicated in these patients because the prepuce is often interposed during the urethral reconstruction, whether as a vascularized flap or free graft. Therefore, establishing a diagnosis during the neonatal period is essential to prevent removing the available and useful preputial tissue with a circumcision. A thorough genitourinary examination of a patient with hypospadias would show two urethral openings: 1) a blind-ending urethral pit at the normal glanular location and 2) an opening of the true urethral meatus on the ventral aspect of the penis (Figure 2). Other classic characteristics associated with hypospadias that may or may not be present include a dorsal hooded prepuce as a result of ventral foreskin deficiency and ventral chordee, or penile curvature. Once the diagnosis is established, the physician should further evaluate the patient for other associated congenital anomalies related to syndromic causes.31 However, approximately 90% of cases are isolated hypospadias.31 The presence of both bilateral cryptorchidism and hypospadias is suggestive of a disorder of sexual differentiation (DSD), though this is considered more common in boys with proximal hypospadias.32 Regardless, any patient with hypospadias and bilateral cryptorchidism should be evaluated with a karyotype, pelvic ultrasound, and serum electrolytes to screen for salt-wasting secondary to congenital adrenal hyperplasia (CAH).
During urologic consultation, several factors should be considered when contemplating repair. A thorough genitourinary examination by an experienced pediatric urologist should be conducted to determine the meatal location, glans volume, penile length, degree of chordee, presence of penoscrotal transposition, and depth and width of the urethral plate. Urinary tract imaging is neither indicated nor necessary for isolated hypospadias.31 These operative characteristics, in addition to a surgeon’s preference and experience, play a role in planning for repair. Last, the risks and benefits of surgery should be discussed thoroughly with the parents to allow for shared-decision making.
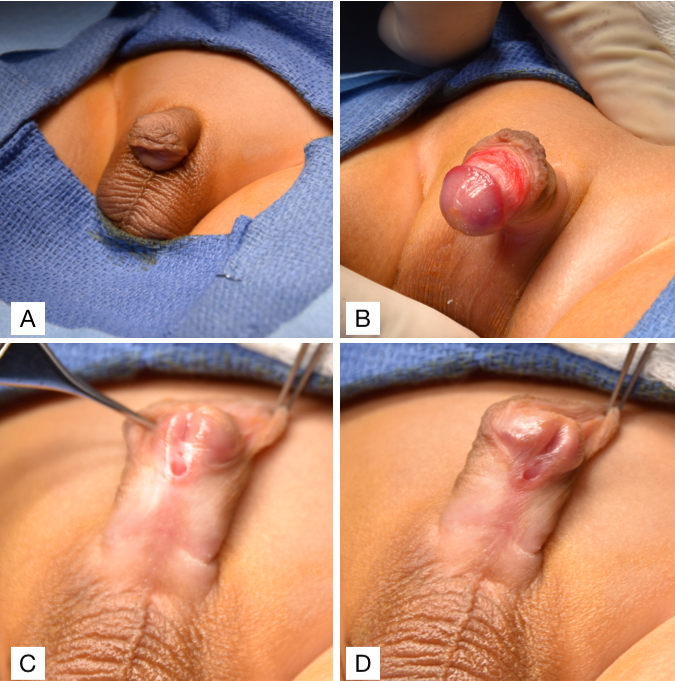
Figure 2 (A) Ventral chordee with a dorsal hooded prepuce. (B) Ventral chordee. (C & D) Sub-coronal hypospadias with a dorsal hooded prepuce.
Preoperative Planning and Management
For most distal hypospadias, surgical repair is not required, especially in glanular hypospadias where the urine stream is straight. Many of these boys have normal voiding and future sexual function of their penis. The “normal” meatal location is widely considered as the distal tip of the glans. Great variation of meatal location has been reported with preservation of sexual and voiding function throughout adulthood in men with untreated hypospadias.33,34 A survey found that men with untreated “milder” or distal hypospadias report difficulties with sexual intercourse associated to curvature, but no significant urinary issues compared to normal men.35 This suggests that distal hypospadias repair should focus on chordee correction and avoid urethroplasty and its associated complications.43 Additionally, infertility is considered to be an issue in proximal hypospadias, not distal.34 However, patients with coronal and mid-penile hypospadias may have urinary spraying or deflection of the urine stream that lead them to sit down to void, or cosmetic issues that cause future psychosexual implications. As such, many parents elect for surgical repair of their child’s distal hypospadias.
As for timing of the surgery, most pediatric urologists agree that repair can be performed starting at 6 months of age. This coincides with the 1996 guidelines from the American Academy of Pediatrics Section of Urology action committee, who report 6–12 months as an acceptable age for hypospadias repair.44 When opting for multi-stage procedures, though this is uncommon in distal hypospadias, 6 months is recommended between procedures to allow for proper wound healing. Notably, efforts should be made to limit a child’s time under anesthesia, as emerging literature express concern for the effect of prolonged anesthesia on brain development in children under 3 years old.36
There is debate among pediatric urologists over the use of preoperative androgen stimulation in boys with hypospadias. Many urologists advocate for its use in boys with severe hypospadias in order to increase phallus length, glans circumference, penile vascularity, and tissue robustness to optimize penile characteristics for repair. However, a systematic review suggests that preoperative hormonal stimulation is associated with increased postoperative complication rates in patients with proximal hypospadias, though it did not reach statistical significance.37 When deemed necessary, we use two doses of 25 mg intramuscular depo-testosterone injections with a six-week interval between doses.
Antibiotic and Analgesic Prophylaxis
Antibiotic prophylaxis is commonly used among pediatric urologists in an effort to minimize the risk of surgical site and urinary tract infections, especially in stented repairs.38 However, concerns over increased rates of antibiotic resistance have raised questions regarding the effectiveness of antibiotic prophylaxis. Retrospective studies have found no significant difference in surgical site and urinary tract infections with and without the use of preoperative antibiotic prophylaxis in hypospadias repair.39,40 However, additional studies with larger sample sizes would be helpful in further clarifying the effectiveness of this practice. At our institution, we use cefazolin (30 mg/kg) intravenously as preoperative antibiotic prophylaxis upon anesthesia induction. As for postoperative antibiotic prophylaxis, a reported 91% of pediatric urologists prescribe postoperative antibiotics when a urethral catheter is left in place.38 However, a recent meta-analysis assessing the effects of postoperative antibiotic prophylaxis after hypospadias repair found limited utility in preventing infectious and wound healing complications, although the risk of bias was high in many of the studies.41 There is currently an ongoing international, multi-institution, randomized controlled trial assessing post-operative prophylaxis.42 At our institution, we prescribe oral TMP-SMX 2 mg/kg daily for the duration of the catheter.
Providing patients with adequate analgesia is especially important in this population since many boys that undergo hypospadias repair are too young to verbally communicate. Various analgesic techniques are used in hypospadias repair including caudal block and peripheral nerve blocks (dorsal nerve penile block and pudendal nerve block). There is ongoing debate over the efficacy of these different techniques and their effects on postoperative outcomes. A meta-analysis of randomized controlled trials and observational studies comparing caudal vs peripheral nerve blocks reports no significant difference in additional analgesic use within 24 hours of surgery, but lower pain scores 24 hours after the surgery with a caudal block.43 The authors also report no additional risk of postoperative complications with caudal blocks.43 On the contrary, a different meta-analysis found a significant association between caudal block analgesia and postoperative complications following hypospadias repair, although these data were likely confounded by hypospadias severity.44 Additional clarifying data is needed to determine the most effective and safest analgesic technique. At our institution, the surgeon prefers a preoperative caudal block for pain control.
Distal Hypospadias Repair
Hypospadias repair should aim to achieve the following three main objectives: ability to comfortably void in an upright position, an appropriate voiding stream, and “normal” penile appearance and function.45 Though there is normal variation in penile appearance, the typical goal is to create a vertical, slit-like meatus at the distal glanular tip.46 Most, if not all, distal hypospadias repair can accomplish these objectives when using the tubularized incised plate (TIP) repair.47,48 The original Thiersch-Duplay repair technique was complemented and popularized by the TIP technique first described by Snodgrass in 1994.47,49 His modification consisted of a deep longitudinal midline incision of the dorsal urethral plate, which allows for widening of the urethral plate to facilitate tubularization. These are the steps performed at our institution, which is a variation of the TIP technique (Recommended Video, (Figure 3), and (Figure 4)50,51
- Insert 5-Fr feeding tube across the hypospadic meatus, place stay sutures on both sides of the future distal meatus, and place the penile tourniquet.
- Create vertical incisions on each edge of the urethral plate, then a crossing incision proximal to the meatus connecting the two.
- Separate the glans flap and make a dorsal midline incision of the urethral plate.
- Urethroplasty is performed using 6-0 PDS suture for a one-layer subepithelial closure with continuous sutures over the 5-French feeding tube.
- A lateral dartos flap is harvested and used to cover the suture line.
- Glansplasty is completed by ventrally re-approximating the glans flaps.
- Deglove the penis to the base.
- Circumcise the excess preputial skin and measure the degree of chordee.
- Re-approximate the ventral penile shaft skin.
- Secure the feeding tube to the glans and apply a thin, compressive, waterproof, and sterile hydrocolloid film followed by a transparent, thin, waterproof, and sterile adhesive wrap dressing to keep the penis elevated (Figure 3)
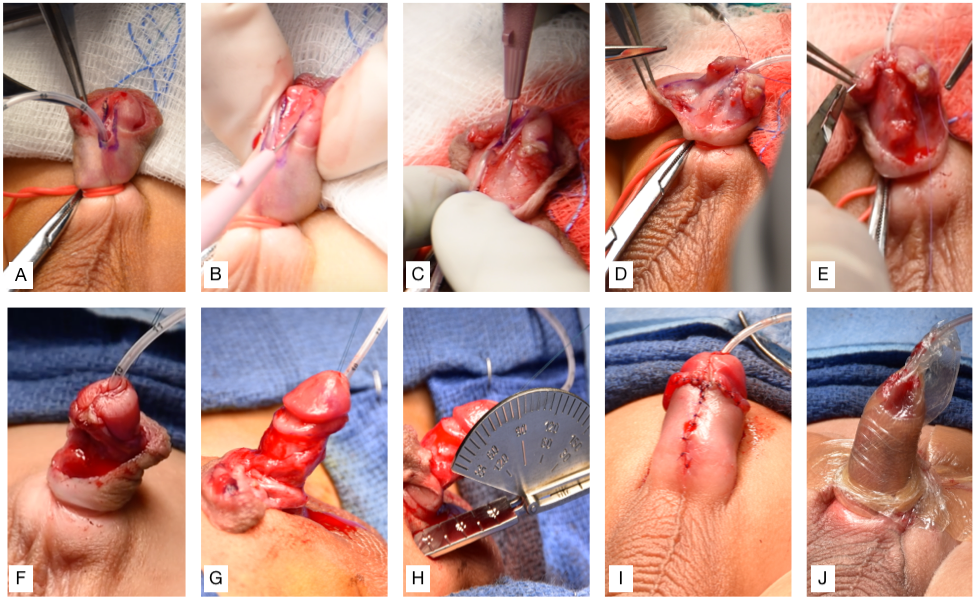
Figure 3 (A) Inserting 5-Fr feeding tube across the hypospadic meatus, placing stay sutures on both sides of the future distal meatus, and placing the penile tourniquet. (B) Vertical incisions on each edge of the urethral plate, then crossing incision connecting the two. (C) Separation of glans flap and midline incision of the urethral plate. (D) Urethroplasty. (E) Dartos flap is used to cover the suture line. (F) Glansplasty is completed by re-approximating glans flap. (G) Degloving the penis. (H) Circumcision and assessment of the degree of chordee using a goniometer. (I) Re-approximation of ventral skin. (J) Securing the feeding tube to the glans and dressing applied to keep the penis elevated.
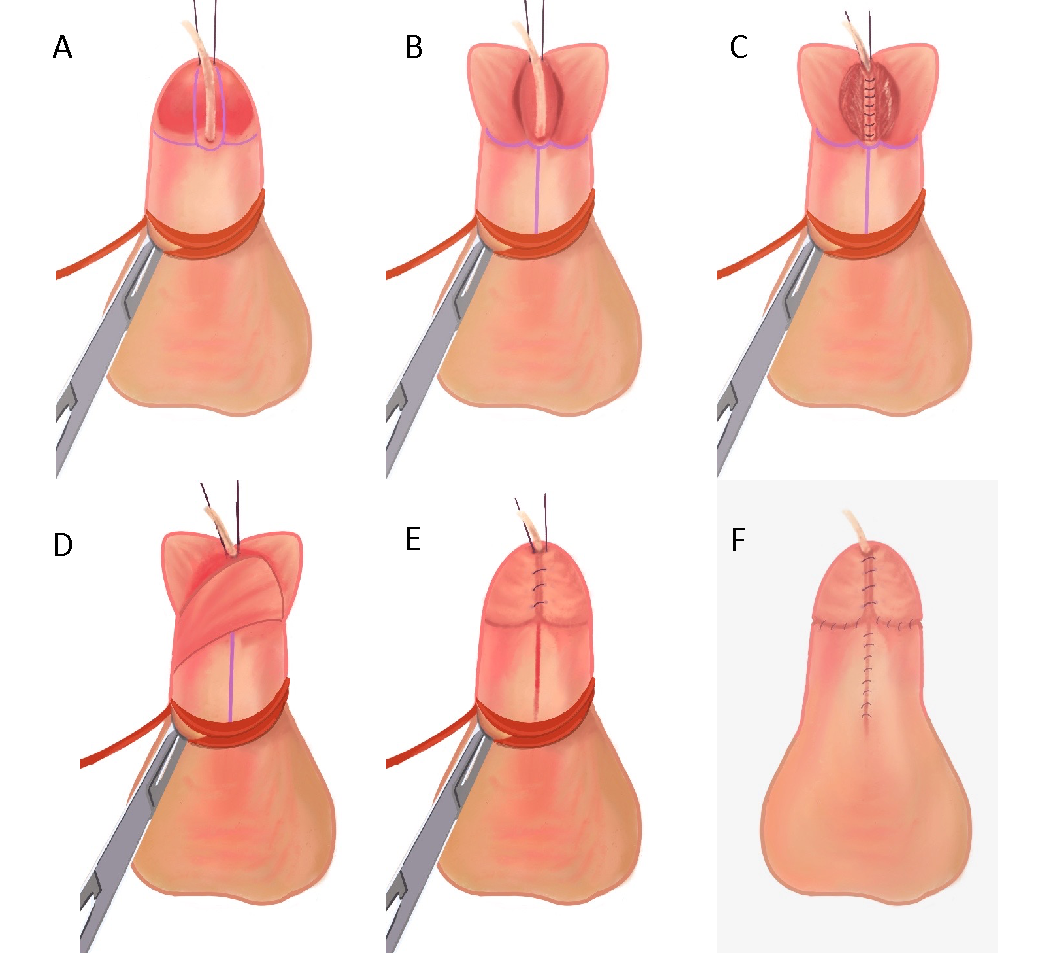
Figure 4 (A) 5-Fr feeding tube inserted across the hypospadic meatus, stay sutures placed on both sides of the future distal meatus, and penile tourniquet in place. (B) Vertical incisions on each edge of the urethral plate, then crossing incision connecting the two. Glans flap are separated and midline urethral plate is incised. (C) Urethroplasty performed with 6-0 PDS suture for a one-layer subepithelial closure with continuous sutures over the 5-French feeding tube. (D) Dartos flap is used to cover the suture line. (E) Glansplasty is completed by re-approximating glans flap. (F) Re-approximation of ventral skin after degloving and circumcision.
The tubularized incised plate technique is widely accepted as the method of choice for distal hypospadias repair. Hypospadias repair at our institution are performed using a variation of the TIP technique, in which the dartos flap is harvested from the lateral rather than dorsal aspect of the penis, as this is our preferred method. Other techniques such as the Mathieu procedure, Duckett onlay island flap repair, and MAGPI are also options. However, the TIP approach has been consistently reported to have low complication rates, especially among high volume surgeons.52,53 In addition to low complication rates, cosmetic results after TIP have been assessed and rated better when compared to the Mathieu and Duckett onlay island flap repairs.46 The favorable cosmesis of the TIP approach is attributed to the creation of a vertical slit-like urethral orifice, as opposed to a circular urethral orifice with the Mathieu procedure. There is also variation in urethroplasty suture techniques within the TIP procedure, which may be performed using either interrupted- or continuous-sutures. Comparative studies of interrupted vs continuous-suture urethroplasty techniques in distal hypospadias repair found no significant difference in complication rates, therefore leaving the choice to surgeon preference.48,54
Recommended Video
Post-Operative Care and Follow-Up
Hypospadias repair at our institution is performed as an outpatient procedure and patients are discharged the same day. We catheterize all our patients with a soft 5-French feeding tube and secure it to the glans with a stay suture, which stays in place for 5–7 days. There is question whether hypospadias repair should be performed stented, as it is classically done for urinary diversion, or non-stented, to prevent bladder spasm and stent removal discomfort. A meta-analysis of randomized controlled trials and cohort studies compared early (bladder spasm, dysuria, UTI, stent failure, urine extravasation, bleeding, urinary retention, et cetera) and late (meatal stenosis, stricture, fistula, diverticulum, glans dehiscence, and other reoperation rates) complication rates after stented vs non-stented distal hypospadias TIP repair.55 There were no significant differences in either early or late complication rates between stented and non-stented TIP repairs in the overall meta-analysis and RCT pool, however the evidence was deemed to be of low-moderate quality. Further RCTs are needed to truly determine its impact in distal hypospadias repair.
Dressing is often used to optimize postoperative healing by promoting immobilization, surgical site protection, tissue adherence, and compression.56 Currently, there is no consensus on the most effective dressing type, or whether applying a dressing is beneficial at all. A randomized trial found no significant difference in complication rates or clinical outcomes when comparing dressed and non-dressed patients following hypospadias repair.56 Of note, there were significantly increased postoperative calls by parents in the non-dressed group. We apply a compressive hydrocolloid dressing followed by an adhesive wrap that keeps the penis elevated to minimize edema. In the case of pretoilet-trained patients, we recommend double diapers while the catheter is in place. We make a hole in the inner diaper to allow the catheter to pass through and drain into the outer diaper. The catheter and dressing are removed at the patient’s first post-operative follow-up visit after 5–7 days. If the patient is toilet-trained, either we keep the feeding tube and drain into pull-ups or use a suprapubic catheter for urine drainage, but never place a Foley catheter. We prescribe oral TMP-SMX 2 mg/kg daily for the duration of the catheter. We also prescribe oxybutynin 0.2 mg/kg twice daily until the catheter is removed to prevent bladder spasms.
The preoperative caudal nerve block helps ameliorate postoperative pain. Further pain control is generally achieved with low doses of NSAID analgesics. While there is variation in opioid prescription for children after hypospadias repair, pediatric urologists are trending away from this practice. A quality improvement initiative using education and a predetermined non-opioid pain regimen was successful in reducing opioid prescriptions following hypospadias repair.57 They found equivalent pain and function outcomes using alternating acetaminophen and ibuprofen, suggesting opioids may be safely omitted while still achieving adequate postoperative pain control.57 None of our patients receive opioids, as we aim to minimize opioid exposure in children.
Our post-operative follow-up schedule is to return to clinic at one week (for dressing removal), and then again at one, three, six, and twelve months Patients are also scheduled to return to clinic at least once after toilet training. We emphasize the importance of post-operative follow-up to the parents in order to optimize outcomes and patient satisfaction, especially since complications may present many years after repair.58
Complications
A majority of patients that undergo distal hypospadias repair have successful surgical outcomes. However, some patients may develop post-operative complications that warrant re-intervention. Early complications that can occur include bleeding, infection, dehiscence, catheter dislodgment, and urinary obstruction. Later complications that may develop are urethrocutaneous fistula, meatal stenosis, glans dehiscence, neourethral stricture, urethral diverticulum, and balanitis xerotica obliterans (BXO).50
A systematic review comprising of 1872 patients who underwent distal hypospadias repair using the TIP technique in 15 case series found a urethrocutaneous fistula rate of 3.8%, meatal stenosis rate of 3.1%, and neourethral stricture rate of 0%.59 Another systematic review of 624 patients that underwent distal hypospadias repair using the TIP technique in 16 studies found a urethrocutaneous fistula rate of 11.1%, meatal stenosis rate of 6.7%, and dehiscence rate of 4.2%.60 Complications such as these often require surgical re-intervention in order to prevent worsening severity of the issue. Notably, primary distal repairs have lower rates of re-operation when compared to secondary and primary proximal repairs.61 When planning for re-operation, it is important to wait at least 6 months from the initial operation to allow for proper tissue healing.
Key Points
- Hypospadias is one of the most common congenital anomalies in newborn males, occurring in about 1:200-300 live births.
- Disrupted urethral fold fusion results in the urethral opening being located on the ventral aspect of the penis.
- Two-hit hypothesis: environmental exposure to endocrine disruptors potentiate the effects of genetic predisposition to hypospadias.
- Two urethral openings on examination: 1) a blind-ending urethral pit at the normal glanular location and 2) an opening of the true urethral meatus on the ventral aspect of the penis.
- Other classic characteristics associated with hypospadias include a dorsal hooded prepuce and ventral chordee.
- Hypospadias repair may be performed starting at 6 months of age.
- The tubularized incised plate (TIP) technique is widely accepted as the method of choice for distal hypospadias repair.
- The most common complications from hypospadias repair include urethrocutaneous fistula, meatal stenosis, glans dehiscence, and neourethral stricture, though rates are low for distal repair.
Conclusion
Hypospadias is one of the most common congenital anomalies in newborn males. It is due to arrested penile development that results in failure of proper urethral fold fusion. Hypospadias is attributed to a multifactorial etiology that derives from genetic predisposition and environmental exposures. A thorough history and examination should be performed to adequately establish a diagnosis of hypospadias and evaluate the patient’s risk characteristics. Surgical repair can be performed as early as 6 months of age and is typically done using the tubularized incised plate technique. Though distal hypospadias repair is consistently reported to have excellent outcomes, long-term follow-up is necessary to monitor for complications and ensure patient satisfaction.
Resources for Patients
Suggested Readings
- HJR H, LL W. Hypospadias, all there is to know. Eur J Pediatr 2017; 176 (4): 435–441. DOI: 10.1007/s00431-017-2864-5.
- Baskin L. What Is Hypospadias? Clin Pediatr (Phila. 2017; 56 (5): 409–418.
- Snodgrass WT, Bush NC, Wein AJ, Kavoussi LR, Partin AW, Peters C. Chapter 147. Hypospadias. Eleventh, Philadelphia, PA: Elsevier; 2016.
References
- Laios K, Karamanou M, Androutsos G. A unique representation of hypospadias in ancient Greek art. Can Urol Assoc J 2012; 6 (1). DOI: 10.5489/cuaj.382.
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. Journal of Pediatric Surgery 2006; 41 (3): 463–472. DOI: 10.1016/j.jpedsurg.2005.11.059.
- Schneuer FJ, Holland AJA, Pereira G, Bower C, Nassar N. Prevalence, repairs and complications of hypospadias: an Australian population-based study. Arch Dis Child 2015; 100 (11): 1038–1043. DOI: 10.1136/archdischild-2015-308809.
- Newborn Clinical Guideline - Hypospadias [Internet. .
- Blaschko SD, Cunha GR, Baskin LS. Molecular Mechanisms of External Genitalia Development. Differentiation 2012; 84 (3): 261–268. DOI: 10.1016/j.diff.2012.06.003.
- Baskin L, Shen J, Sinclair A. Development of the Human Penis and Clitoris. Differentiation 2018; 103: 74–85. DOI: 10.1016/j.diff.2018.08.001.
- Cunha GR, Liu G, Sinclair A. Androgen-independent events in penile development in humans and animals. Differentiation 2020; 111: 98–114. DOI: 10.1016/j.diff.2019.07.005.
- Liu X, Liu G, Shen J. Human Glans and Preputial Development. Differentiation 2018; 103: 86–99. DOI: 10.1016/j.diff.2018.08.002.
- Li Y, Sinclair A, Cao M. Canalization of the Urethral Plate Precedes Fusion of the Urethral Folds during Male Penile Urethral Development: The Double Zipper Hypothesis. J Urol 2015; 193 (4): 1353–1360. DOI: 10.1016/j.juro.2014.09.108.
- Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res 2001; 305 (3): 379–387. DOI: 10.1007/s004410000345.
- Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect 2001; 109 (11): 1175–1183. DOI: 10.1289/ehp.011091175.
- Baskin L. What Is Hypospadias? Clin Pediatr (Phila. 2017; 56 (5): 409–418.
- Bergman J, Loane M, Vrijheid M. Epidemiology of hypospadias in Europe: a registry-based study. World Journal of Urology 2015; 33. DOI: 10.1007/s00345-015-1507-6.
- Li Y, Mao M, Dai L. Time trends and geographic variations in the prevalence of hypospadias in China. Birth Defects Research Part A: Clinical And Molecular Teratology 2012; 94 (1): 36–41. DOI: 10.1002/bdra.22854.
- S CK, S KK, Y PH. Trends in the incidence of cryptorchidism and hypospadias of registry-based data in Korea: a comparison between industrialized areas of petrochemical estates and a non-industrialized area. Asian J Androl 2011; 13 (5): 715–718. DOI: 10.1038/aja.2010.53.
- Springer A, Heijkant M, Baumann S. Worldwide prevalence of hypospadias. Journal of Pediatric Urology 2016; 12 (3). DOI: 10.1016/j.jpurol.2015.12.002.
- Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias Trends in Two US Surveillance Systems. Pediatrics 1997; 100 (5): 831–834. DOI: 10.1016/s0022-5347(01)63643-7.
- Porter MP, Faizan MK, Grady RW, Mueller BA. Hypospadias in Washington State: maternal risk factors and prevalence trends. Pediatrics 2005; 115 (4). DOI: 10.1542/peds.2004-1552.
- Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, Gier RPE, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr 2007; 166 (7): 671–678. DOI: 10.1007/s00431-006-0304-z.
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005; 159 (10): 957–962. DOI: 10.1016/s0084-4071(08)70428-7.
- Baskin LS. Can we prevent hypospadias? Journal of Pediatric Urology 2007; 3 (6): 420–425. DOI: 10.1016/j.fertnstert.2007.12.024.
- Carmichael SL, Ma C, Choudhry S, Lammer EJ, Witte JS, Shaw GM. Hypospadias and genes related to genital tubercle and early urethral development. J Urol 2013; 190 (5): 1884–1892. DOI: 10.1016/j.juro.2013.05.061.
- Ollivier M, Paris F, Philibert P. Family History is Underestimated in Children with Isolated Hypospadias: A French Multicenter Report of 88 Families. J Urol 2018; 200 (4): 890–894. DOI: 10.1016/j.juro.2018.04.072.
- LFM Z, LM, WFJ F, B F, M KNV, N R. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update 2012; 18 (3): 260–283. DOI: 10.1093/humupd/dms002.
- HJR H, LL W. Hypospadias, all there is to know. Eur J Pediatr 2017; 176 (4): 435–441. DOI: 10.1007/s00431-017-2864-5.
- Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect 1999; 107 (4): 297–302. DOI: 10.1289/ehp.99107297.
- Lund L, Engebjerg MC, Pedersen L, Ehrenstein V, Nørgaard M, Sørensen HT. Prevalence of hypospadias in Danish boys: a longitudinal study, 1977-2005. Eur Urol 2009; 55 (5): 1022–1026. DOI: 10.1016/s8756-5005(09)79126-7.
- Abdullah NA, Pearce MS, Parker L, Wilkinson J JR, B MN, R.J.Q.. Birth prevalence of cryptorchidism and hypospadias in northern England, 1993-2000. Arch Dis Child 2007; 92 (7): 576–579. DOI: 10.1136/adc.2006.102913.
- Nassar N, Bower C, Barker A. Increasing prevalence of hypospadias in Western Australia, 1980-2000. Arch Dis Child 2007; 92 (7): 580–584. DOI: 10.1136/adc.2006.112862.
- Choudhry S, Baskin LS, Lammer EJ. Genetic polymorphisms in ESR1 and ESR2 genes, and risk of hypospadias in a multiethnic study population. J Urol 2015; 193 (5): 1625–1631. DOI: 10.1016/j.juro.2014.11.087.
- Kalfa N, Liu B, Klein O, Wang M-H, Cao M, Baskin LS. Genomic variants of ATF3 in patients with hypospadias. J Urol 2008; 180 (5): 2188.
- Liu B, Wang Z, Lin G. Activating transcription factor 3 is up-regulated in patients with hypospadias. Pediatr Res 2005; 58 (6): 1280–1283. DOI: 10.1203/01.pdr.0000187796.28007.2d.
- Wang Z, Liu BC, Lin GT. Up-Regulation of Estrogen Responsive Genes in Hypospadias: Microarray Analysis. The Journal of Urology 2007; 177 (5): 1939–1946. DOI: 10.1016/j.juro.2007.01.014.
- Qiao L, Tasian GE, Zhang H. Androgen receptor is overexpressed in boys with severe hypospadias, and ZEB1 regulates androgen receptor expression in human foreskin cells. Pediatr Res 2012; 71 (4 Pt 1): 393–398. DOI: 10.1016/j.yuro.2012.07.021.
- Kojima Y, Koguchi T, Mizuno K. Single Nucleotide Polymorphisms of HAAO and IRX6 Genes as Risk Factors for Hypospadias. J Urol 2019; 201 (2): 386–392.
- Kim K, Liu W, Cunha GR. Expression of the androgen receptor and 5α-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res 2002; 307 (2): 145–153. DOI: 10.1007/s004410100464.
- Edery P. Genetics of hypospadias. Dialogues Pediatr Urol 2007; 28: 3–6. DOI: 10.1016/j.jpurol.2007.01.087.
- Poon S, Koren G, Carnevale A. Association of In Utero Exposure to Polybrominated Diphenyl Ethers With the Risk of Hypospadias. JAMA Pediatr 2018; 172 (9): 851–856. DOI: 10.1001/jamapediatrics.2018.1492.
- Snodgrass WT, Bush NC, Wein AJ, Kavoussi LR, Partin AW, Peters C. Chapter 147. Hypospadias. Eleventh, Philadelphia, PA: Elsevier; 2016.
- Tasian GE, Zaid H, Cabana MD, Baskin LS. Proximal hypospadias and risk of acquired cryptorchidism. J Urol 2010; 184 (2): 715–720. DOI: 10.1016/j.juro.2010.03.056.
- Fichtner J, Filipas D, Mottrie AM, Voges GE, Hohenfellner R. Analysis of meatal location in 500 men: wide variation questions need for meatal advancement in all pediatric anterior hypospadias cases. J Urol 1995; 154 (2 Pt 2): 833–834. DOI: 10.1016/s0022-5347(01)67177-5.
- Dodds PR, Batter SJ, Shield DE, Serels G SR, FA M, P.K.. Adaptation of Adults to Uncorrected Hypospadias. Urology 2008; 71 (4): 682–685. DOI: 10.1016/j.urology.2007.07.078.
- Schlomer B, Breyer B, Copp H, Baskin L, DiSandro M. Do adult men with untreated hypospadias have adverse outcomes? A pilot study using a social media advertised survey. J Pediatr Urol 2014; 10 (4): 672–679. DOI: 10.1016/j.jpurol.2014.01.024.
- Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia. American Academy of Pediatrics; 1996, DOI: 10.1542/peds.97.4.590.
- Adler AC, Chandrakantan A, Sawires Y. Analysis of 1478 Cases of Hypospadias Repair. The Incidence of Requiring Repeated Anesthetic Exposure as Well as Exploration of the Involvement of Trainees on Case Duration. Anesth Analg 2019.
- Wright I, Cole E, Farrokhyar F, Pemberton J, Lorenzo AJ, Braga LH. Effect of preoperative hormonal stimulation on postoperative complication rates after proximal hypospadias repair: a systematic review. J Urol 2013; 190 (2): 652–659. DOI: 10.1016/j.juro.2013.02.3234.
- Hsieh MH, Wildenfels P, Gonzales ET. Surgical antibiotic practices among pediatric urologists in the United States. Journal of Pediatric Urology 2011; 7 (2): 192–197. DOI: 10.1016/j.jpurol.2010.05.001.
- Smith J, Patel A, Zamilpa I. Analysis of preoperative antibiotic prophylaxis in stented, distal hypospadias repair. Can J Urol 2017; 24 (2): 8765–8769.
- Baillargeon E, Duan K, Brzezinski A. The role of preoperative prophylactic antibiotics in hypospadias repair. Can Urol Assoc J 2014; 8 (7-8): 236–240. DOI: 10.5489/cuaj.1838.
- Chua ME, Kim JK, Rivera KC. The use of postoperative prophylactic antibiotics in stented distal hypospadias repair: a systematic review and meta-analysis. Journal of Pediatric Urology 2019; 15 (2): 138–148. DOI: 10.1016/j.jpurol.2018.10.012.
- Cheng EY, Faasse MA. Trimethoprim-sulfamethoxazole vs. Placebo After Hypospadias Repair: a Multicenter, Double-blind. Randomized Trial [Internet] Clinicaltrialsgov 2018.
- Zhu C, Wei R, Tong Y. Analgesic efficacy and impact of caudal block on surgical complications of hypospadias repair: a systematic review and meta-analysis. Reg Anesth Pain Med 2019; 44 (2): 259–267. DOI: 10.1136/rapm-2018-000022.
- Tanseco PP, Randhawa H, Chua ME. Postoperative complications of hypospadias repair in patients receiving caudal block vs. non-caudal anesthesia: A meta-analysis. Can Urol Assoc J 2019; 13 (8). DOI: 10.5489/cuaj.5688.
- Deibert CM, Hensle TW. The psychosexual aspects of hypospadias repair: A review. Arab J Urol 2011; 9 (4): 279–282. DOI: 10.1016/j.aju.2011.10.004.
- Ververidis M, Dickson AP, Gough DCS. An objective assessment of the results of hypospadias surgery. BJU Int 2005; 96 (1): 135–139. DOI: 10.1111/j.1464-410x.2005.05582.x.
- Snodgrass W. Tubularized, incised plate urethroplasty for distal hypospadias. J Urol 1994; 151 (2): 464–465. DOI: 10.1016/s0022-5347(17)34991-1.
- Snodgrass WT, Bush N, Cost N. Tubularized incised plate hypospadias repair for distal hypospadias. Journal of Pediatric Urology 2010; 6 (4): 408–413. DOI: 10.1016/j.jpurol.2009.09.010.
- Snodgrass WT. Snodgrass technique for hypospadias repair. BJU Int 2005; 95 (4): 683–693. DOI: 10.1111/j.1464-410x.2005.05384.x.
- Barashi NS, Gundeti MS. Chapter 15. Surgical Reconstructions of Distal Hypospadias: The University of Chicago Experience. Gundeti MS. Surgical Techniques in Pediatric and Adolescent Urology. Jaypee Brothers,Medical Publishers Pvt. Limited; 2019.
- Herrera O, Mostafa S, Lomba T. A 10-step procedure for distal hypospadias repair with 50% resident involvement is safe and effective. Urology Video Journal 2021; 11 (100094). DOI: 10.1016/j.urolvj.2021.100094.
- Nguyen MT, Snodgrass WT, Zaontz MR. Effect of urethral plate characteristics on tubularized incised plate urethroplasty. J Urol 1262; 2004;171(3):1260-2. DOI: 10.1097/01.ju.0000110426.32005.91.
- Braga LHP, Lorenzo AJ, Salle JLP. Tubularized incised plate urethroplasty for distal hypospadias: A literature review. Indian J Urol 2008; 24 (2): 219–225. DOI: 10.4103/0970-1591.40619.
- Gupta A, Gupta R, Srivastav P. Comparison of interrupted- and continuous-suture urethroplasty in tubularised incised-plate hypospadias repair: A prospective study. Arab J Urol 2017; 15 (4): 312–318. DOI: 10.1016/j.aju.2017.10.004.
- Chua M, Welsh C, Amir B. Non-stented versus stented urethroplasty for distal hypospadias repair: A systematic review and meta-analysis. Journal of Pediatric Urology 2018; 14 (3): 212–219. DOI: 10.1016/j.jpurol.2017.11.023.
- JG S, LG P, BL S. A prospective randomized trial of dressings versus no dressings for hypospadias repair. Journal of Urology 2000; 164 (3 Part 2): 981–983. DOI: 10.1016/s0022-5347(05)67231-x.
- O’Kelly F, Pokarowski M, DeCotiis KN. Structured opioid-free protocol following outpatient hypospadias repair - A prospective SQUIRE 2.0-compliant quality improvement initiative. Journal of Pediatric Urology 2020; 16 (5). DOI: 10.1016/j.jpurol.2020.06.012.
- Lucas J, Hightower T, Weiss DA. Time to Complication Detection after Primary Pediatric Hypospadias Repair: A Large, Single Center, Retrospective Cohort Analysis. J Urol 2020; 204 (2): 338–344. DOI: 10.1097/ju.0000000000000762.
- Wilkinson DJ, Farrelly P, Kenny SE. Outcomes in distal hypospadias: A systematic review of the Mathieu and tubularized incised plate repairs. Journal of Pediatric Urology 2012; 8 (3): 307–312. DOI: 10.1016/j.jpurol.2010.11.008.
- Zhang Y, Shen Z, Zhou X. Comparison of meatal-based flap (Mathieu) and tubularized incised-plate (TIP) urethroplasties for primary distal hypospadias: A systematic review and meta-analysis. Journal of Pediatric Surgery 2020. DOI: 10.1016/j.jpedsurg.2020.03.013.
- Pfistermuller KLM, McArdle AJ, Cuckow PM. Meta-analysis of complication rates of the tubularized incised plate (TIP) repair. J Pediatr Urol 2015; 11 (2): 54–59. DOI: 10.1016/j.jpurol.2014.12.006.
Ultima atualização: 2023-02-22 15:40