09: Basics of Pediatric Endoscopy
Este capítulo levará aproximadamente 19 minutos para ler.
Introduction
The advent of endoscopic instrumentation has revolutionized the field of urology, improving both diagnostic and therapeutic potential for urologic disorders. Over the past several decades, technologic advancements have allowed miniaturization of adult urologic instruments for use in the pediatric genitourinary tract. Cystourethroscopy, ureteroscopy and nephroscopy are indispensable instruments for the pediatric urologist to diagnose and treat conditions from vesicoureteral reflux, nephrolithiasis and posterior urethral valves to congenital anomalies and genitourinary trauma. Previously open surgical procedures have been transformed into endoscopic procedures that are less invasive and allow faster recovery of our patients.
In this chapter, we will discuss the role of lower and upper tract endoscopy in the pediatric patient and provide a guide for evaluation, diagnosis, and treatment of conditions requiring pediatric endoscopy.
History of Endourology
Prior to the arrival of endoscopic instruments, surgical procedures involving bodily orifices were limited to use of specula, probes, and knives.1 The first endoscopic prototype was introduced by Phillip Bozzini in 1806. The ‘lichtleiter’ or ‘light conducter’ consisted of a long funnel placed in a sharkskin covered box and a candle for illumination. Angled mirrors inside the box directed the light into the human body. It was used to examine the vagina, bladder, and nasopharynx, but was never used inside a patient.1,2,3,4 In 1853, Antoine Desormeaux introduced the first working cystoscope and is credited as the ‘father of cystoscopy’.2,3,4 Technologic advances included a smaller endoscope, a kerosene lamp, and a concave mirror with a central hole that improved device illumination.2 He is the first known to use his primitive cystoscope on a patient for excision of a urethral papilloma.4 Major complications included thermal burns associated with the kerosene light source and insufficient illumination.5 In 1877, German urologist Maximilian Nitze utilized a miniaturized telescope with a series of lenses along a hollow tube to magnify the image and a water-cooled electric platinum filament at the end for illumination.6 When Thomas Edison invented the lightbulb in 1878, Nitze’s device was modified by adding a lightbulb to the tip of the cystoscope.2,7 The Amici prism, developed in 1906, allowed images offset by 90˚ to appear in the correct orientation, and this technology was incorporated into cystoscope design thereafter.7
The discovery that glass was a better conductor of light than air paved the way for fiberoptic designs. Harold Hopkins invented the rod lens system in 1951 by bundling and arranging glass fibers 0.1mm in diameter coaxially with the eyepiece to transmit images.7 The ‘fiberoscope’ coupled with an external light source improved light transmission > 80 times, and the system was patented in 1959.7 This technology laid the groundwork for the modern rigid cystoscope.
In addition to endoscopic advances, camera systems have also improved over time. With miniaturization of the camera equipment and invention of a charge-coupling device, optical images obtained from the endoscope are converted into photons and digital images.3,8 Digital images can now be displayed on external monitors, an innovation transformative for urologic surgical education.
Cystourethroscopy
Indications
Cystoscopy serves an important role for the diagnosis and treatment of pediatric genitourinary tract pathology. In addition, anatomic information obtained from cystourethroscopy facilitates planning of genitourinary reconstruction. Common indications include evaluation of congenital genitourinary anomalies (bladder exstrophy-epispadias complex, cloacal anomalies, posterior urethral valves, ectopic ureters), vaginal anatomy, and urinary channels, endoscopic injection for vesicoureteral reflux and treatment of bladder stones.9 Virtually all procedures are performed under general anesthesia in the operating room. Table 1 outlines common indications for pediatric cystourethroscopy. Table 2 offers common treatment interventions.
Table 1 Common indications for pediatric cystourethroscopy.
| Diagnosis | Details |
|---|---|
| Congenital anomalies | Exstrophy-epispadias complex |
| Cloacal anomalies | |
| Ectopic ureters | |
| Vaginoscopy for vaginal anomalies | |
| Retrograde pyelograms for ureteral strictures, UPJ, UVJ obstruction | |
| Malignancy | Surveillance of gastric augmentation |
| Evaluation of bladder or urethral mass | |
| Evaluation of voiding complaints: gross hematuria, weak stream, urinary incontinence | |
| CMG for intraoperative urodynamics | |
| Evaluation of previous reconstructive surgery | Bladder neck procedures |
| Urinary channels |
Table 2 Common cystourethroscopy treatment interventions.
| Treatment |
|---|
| Posterior urethral valves |
| Bladder stone extraction |
| Endoscopic injection for VUR, sphincteric, channel incompetence |
| Ureterocele puncture |
| Placement of suprapubic or urethral catheter |
Equipment
Pediatric and adolescent cystoscopes come in a variety of sizes, in both flexible and rigid models. Instrument size is denoted in the unit French (Fr), with a 1 Fr diameter equivalent to 1/3 mm.10 Rigid cystoscope components include an optical lens, bridge, sheath and obturator. Lenses are manufactured with tip angles ranging from 0–120˚. In our practice, 0˚ and 30˚ lenses are most common. The bridge connects the lens to the sheath and provides 1–2 working channels. Sheath sizes range from 5 Fr to adult sizes and have associated irrigation ports. The obturator can be used in place of the lens inside the sheath to blunt the tip of the sheath for blind passage through the urethra into the bladder. Rigid cystoscopy also requires a light source, endoscopic camera, external monitor and irrigation fluid. Flexible cystoscopes incorporate the endoscope, light source, and camera together and have a single working channel for irrigation and instrument passage. Flexible cystoscope tips can deflect up to 210˚, facilitating device maneuverability, and are available in a variety of sizes.
In our practice, rigid cystoscopes are used in most cases and flexible cystoscopes are reserved for cases when positioning into lithotomy is difficult or for navigation of tortuous catheterizable channels.
Preoperative Considerations
Urinalysis should be performed prior to urinary tract instrumentation and urine culture if applicable.11 Preoperative urine culture and antibiotic treatment, if indicated, is especially important in patients whose urinary tract is likely colonized such as patients on intermittent catheterization or with indwelling catheters. If a urinary tract infection is present, the patient should be treated with culture specific antibiotics and the procedure postponed due to the risk of bacteremia and sepsis.
The AUA Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis in 2019 recommends against preprocedural antibiotics for routine diagnostic cystoscopy in the absence of specific patient risk factors, such as immunocompromise, chronic GU tract colonization, anatomic anomalies predisposing to urinary stasis and poor nutritional status.12 No clear guidance exists specifically for pediatric endourologic instrumentation, but we use these guidelines to direct procedural antibiotic prophylaxis. Detailed surgical history including review of operative reports is essential prior to endoscopy, especially in the setting of previous genitourinary reconstruction.
Surgical Considerations
Prior to induction of anesthesia, endoscopic instruments should be inspected for completeness, compatibility, and function. This is especially important in pediatric endoscopy where various sizes of instruments and accessories are used. Following induction of general anesthesia and preprocedural antibiotic prophylaxis (when indicated), the patient is positioned into dorsal lithotomy (Figure 1). In infants, small rolled towels or gel rolls can be placed under the knees and legs secured in place with tape. Larger children are positioned using stirrups. Genitalia and perineum are prepped with betadine containing antiseptic solution as alcohol and chlorhexidine-based preparations can damage mucus membranes. External anatomy is examined. Mild meatal stenosis can be treated with sequential dilation with McCrae sounds, whereas severe meatal stenosis may require formal meatotomy. Next, a lubricated cystoscope is passed into the urethra and irrigation is turned on. In male patients, the penis is placed on stretch and directed superiorly. This position is optimal for urethroscopy of the anterior urethra, then the surgeon’s hand is dropped inferiorly to allow passage into the posterior urethra and bladder. Systematic evaluation of the bladder is performed, any ancillary interventions performed, and the urethra is visualized with active inflow of irrigation during regress of the cystoscope. Note, inspection of the augmented bladder may be difficult due to mucosal folds and mucus production. Irrigation of mucus out of the bladder and judicious use of irrigation can improve visualization. Overdistension of the augmented bladder should be avoided as this can lead to bladder perforation. The flexible cystoscope is passed per urethra in a similar fashion to a urethral catheter and the tip is deflected as needed for visualization and inspection upon entry into the bladder.
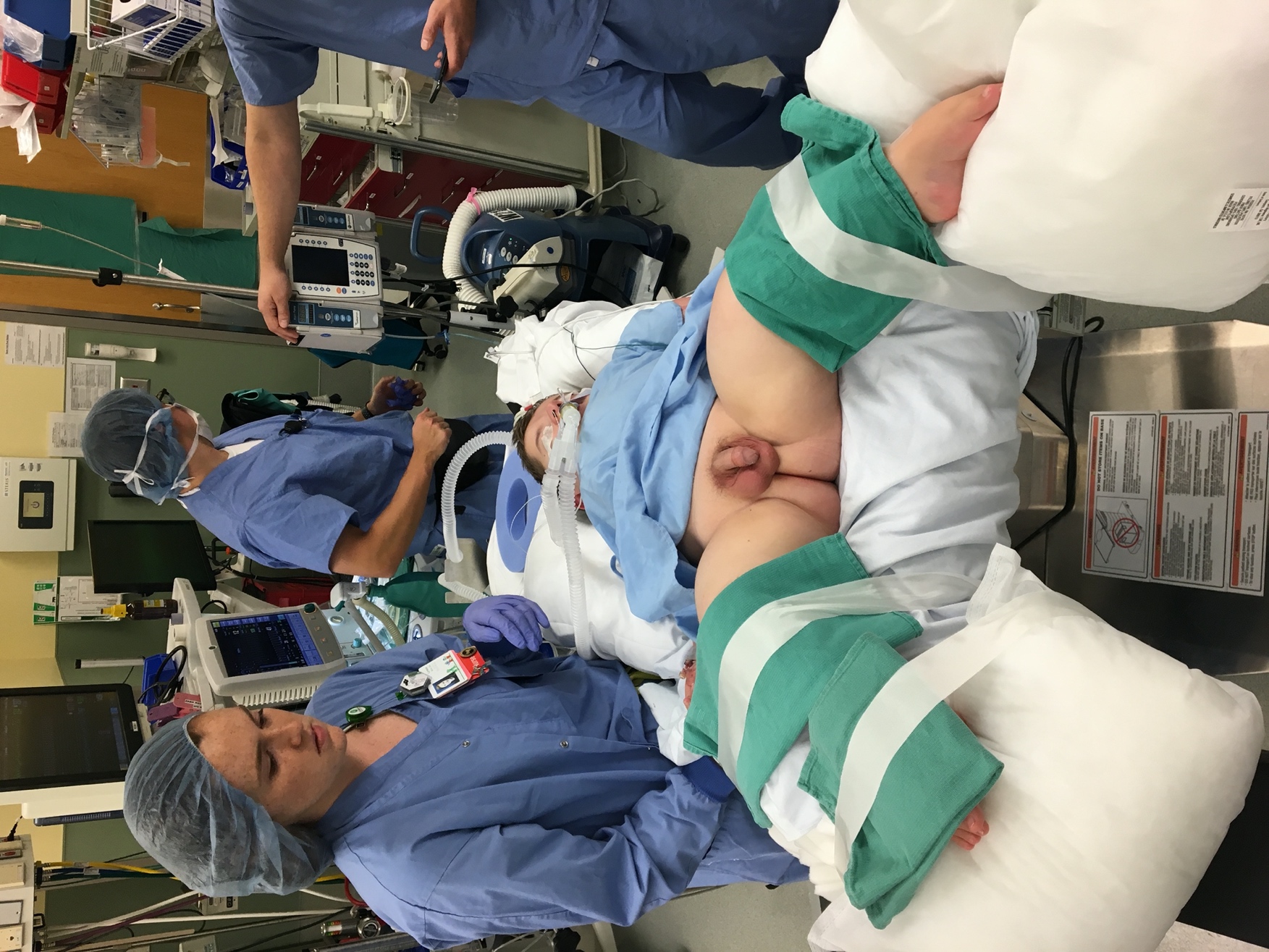
Figure 1 Modified Dorsal Lithotomy. Careful positioning into modified dorsal lithotomy in a patient with osteogenesis imperfecta for ureteroscopic stone extraction.
Cystoscopy can also be performed through a suprapubic tube tract, vesicostomy or catheterizable channel with similar technique. It is important to note surgical details (e.g. type of enteric segment used, channel trajectory, continence mechanism) prior to endoscopy. Catheterizable channels are delicate, and the scope should not be advanced until the lumen is visualized to avoid channel damage or perforation.
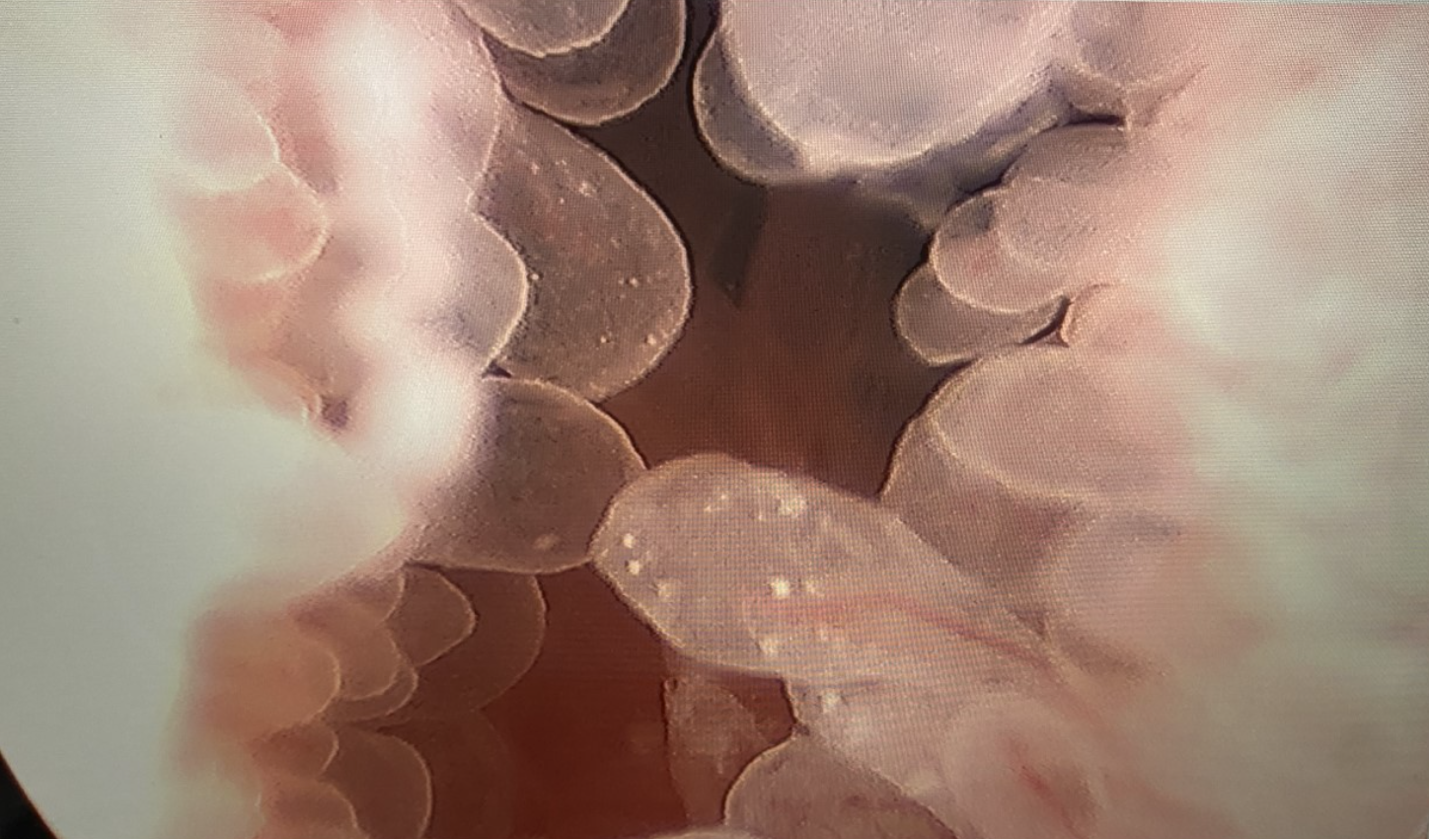
Figure 2 Cystoscopy with Transurethral Resection of Bladder Tumor. Transurethral resection of a papillary bladder neck mass in a teenage male with eosinophilic cystitis.
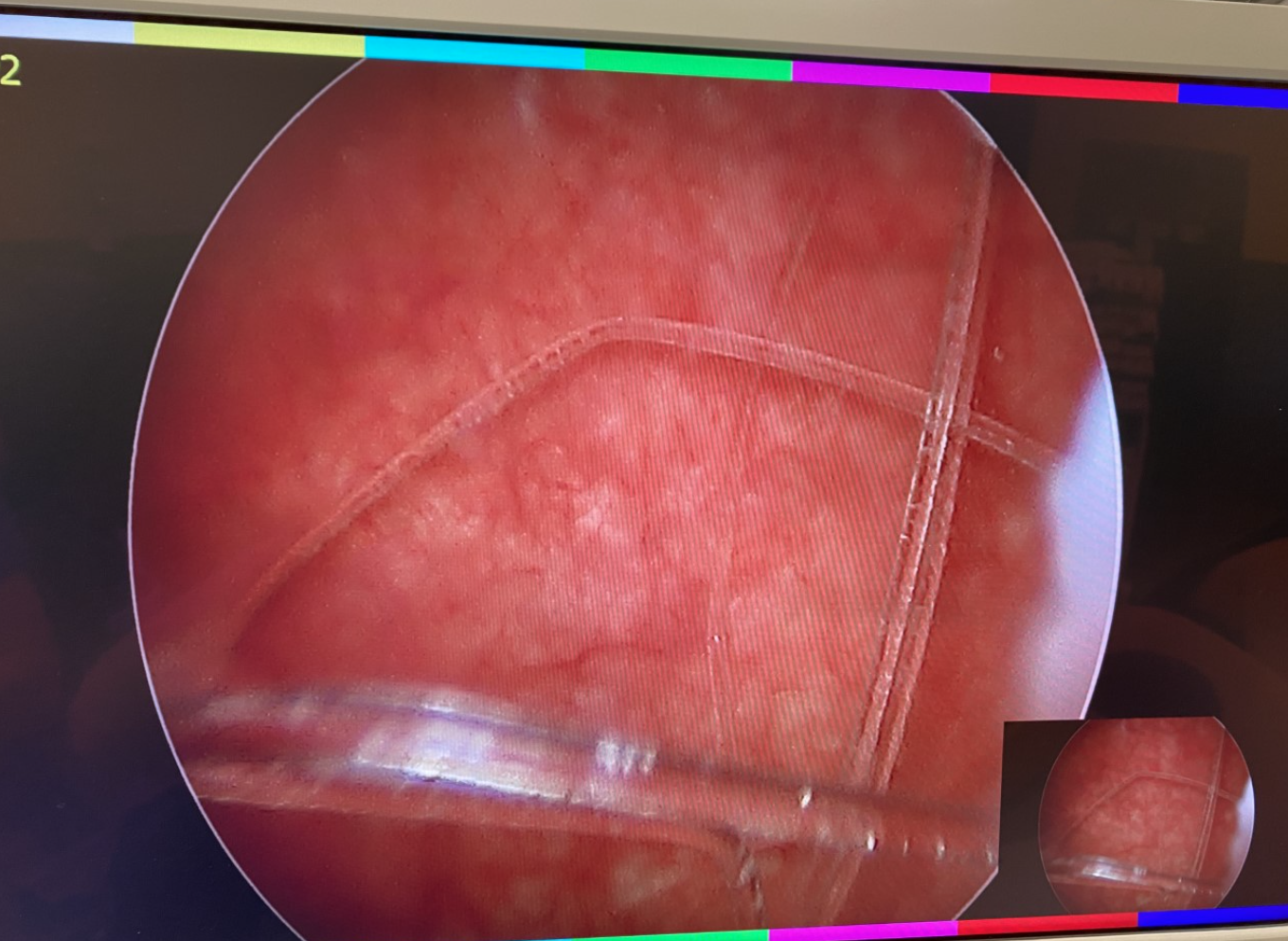
Figure 3 Foreign Body of the Bladder. Cystoscopy with basket extraction of a foreign body of the bladder.
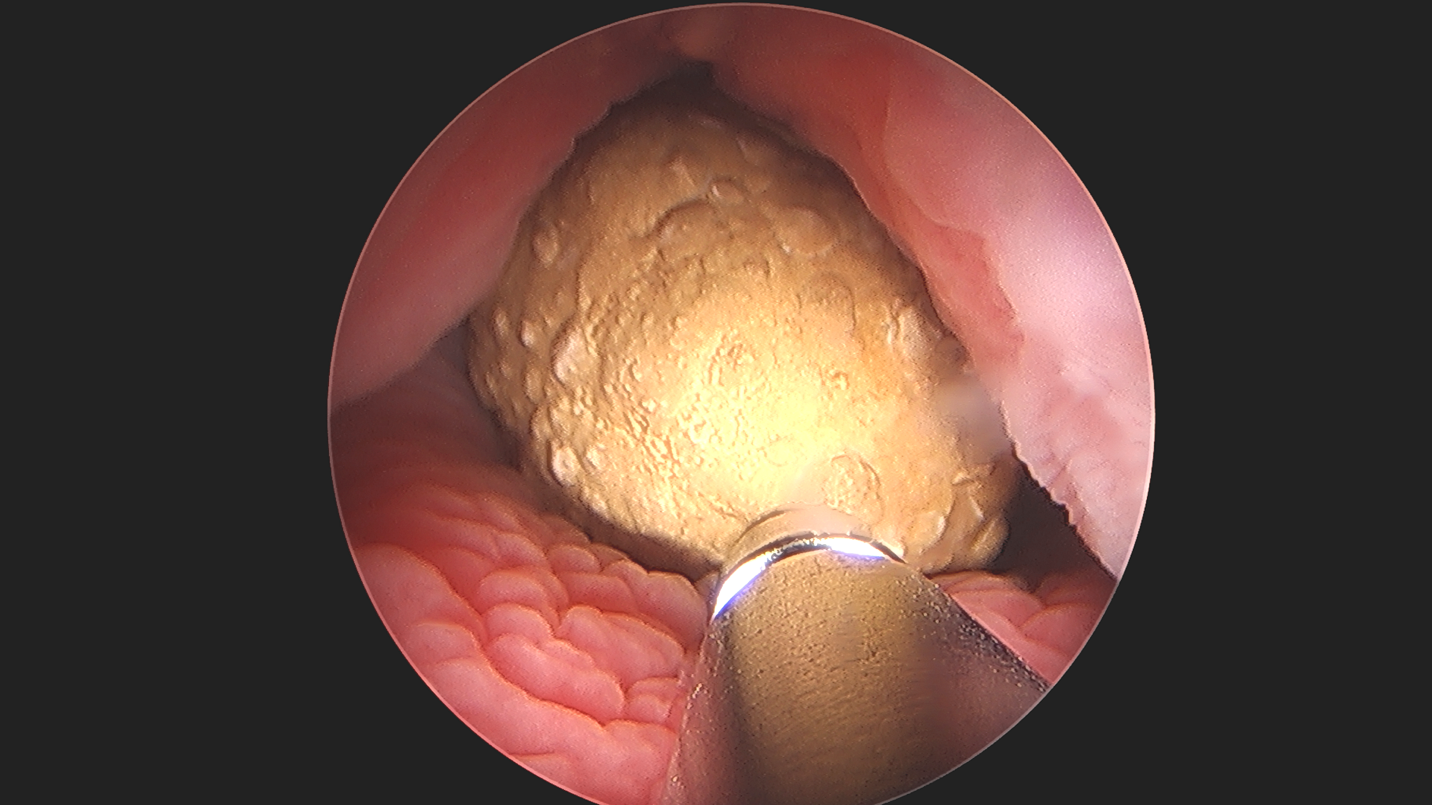
Figure 4 Percutaneous Cystolithotomy. Ultrasonic lithotripsy of large bladder stone in an augmented bladder, demonstrating the applicability and versatility of endoscopy.
Complications
Complications following diagnostic cystourethroscopy are rare and include asymptomatic bacteriuria (5–8%), symptomatic urinary tract infection (2–5%) and gross hematuria.13
Postoperative Care and Follow Up
Cystourethroscopy is typically an outpatient surgical procedure and causes minimal to no discomfort in patients. Unless contraindicated we always apply topical anesthetics at the end of cystourethroscopy (e.g., lidocaine jelly). We do not prescribe post-operative antibiotics, unless the patient possesses risk factors for urosepsis and an endoscopic treatment is performed.12 Continuous antibiotic prophylaxis for vesicoureteral reflux is continued until outpatient follow up after endoscopic injection. Dysuria or other storage lower urinary tract symptoms are treated with acetaminophen or nonsteroidal anti-inflammatories and anticholinergics, if needed. Follow up is determined by patient diagnosis and intervention performed.
Ureteroscopy
Indications
Ureteroscopy provides access to the upper urinary tract for diagnostic and therapeutic purposes. Improvement of optics and miniaturization of ureteroscopes allows ureteral and renal calculi formerly treated with shock wave lithotripsy to be managed ureteroscopically.14 The AUA/Endourology Society 2016 Guidelines on Surgical Management of Stones outlines ureteroscopy as an option for pediatric patients with a total renal stone burden of ≤ 20 mm.15 Additional indications include laser incision for short ureteral strictures, endopyelotomy for recurrent ureteropelvic junction obstruction (in select cases), and evaluation of lateralizing gross hematuria and ureteral filling defects.
Equipment
Ureteroscopes are available in digital and fiberoptic flexible and semirigid models with a variety of sizes ranging from 4.5–11 Fr.16 Semirigid ureteroscopes are smaller as fiberoptics are incorporated into the endoscope and require an external light and camera source. Two channels, one used for irrigation and the other for instrument passage, improves visualization. Semirigid ureteroscopes are used in the ureter distal to the iliac vessels.17 Microureteroscopes such as the 4.5 Fr Ultrathin semirigid ureteroscope (Richard Wolf, Knittlingen, Germany) reduce access failure in small caliber pediatric ureters.18 Flexible ureteroscopes incorporate an optical system, deflection mechanism and working channel. Digital models include the light source and camera built into the endoscope whereas fiberoptic models require an external camera and light source. Distal tip deflection up to 270˚ allows navigation of tortuous ureters and the renal pelvis and calyces.19 The flexible ureteroscope is preferred for use in the ureter proximal to the iliac vessels and the renal pelvis and calyces. The flexible ureteroscope’s deflection mechanism is delicate, and can become damaged with repeated use. Its working channel can sustain damage from instrument passage or inadvertent laser firing within the endoscope, leading to device malfunction.11 Accordingly, single use flexible ureteroscopes are gaining popularity as they demonstrate similar efficacy as conventional flexible ureteroscopes and may offer cost savings.20 At present, their role in the pediatric patient remains to be elucidated.
Ureteroscopy should be performed in an endoscopy suite well equipped with all necessary supplies, fluoroscopy and experienced operating room personnel. Basic equipment necessary for ureteroscopy includes endoscopes (semirigid and flexible), a holmium laser and laser fibers, guide wires, open ended ureteral catheters, dual lumen catheters, coaxial dilators, ureteral access sheaths, baskets and ureteral stents. A variety of sizes of disposable instruments should be available and tailored to the child’s size.
Preoperative Considerations
A urine culture should be performed prior to urinary tract instrumentation. If a urinary tract infection or chronic bacterial colonization is present, the patient should be treated with culture specific antibiotics and the procedure postponed due to the risk of bacteremia and sepsis.11 The AUA Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis in 2019 recommends a single dose of perioperative TMP-SMX or a 1st or 2nd generation cephalosporin for ureteroscopic procedures.12 Although no specific guideline exists specifically for pediatric patients, it is our practice to use cefazolin with or without gentamicin prior to ureteroscopy.
While the goal of ureteroscopy with stone extraction is to achieve complete stone clearance with as few procedures as possible, multiple procedures may be necessary to successfully treat ureteral or renal stones in children. The small caliber of pediatric ureters, especially in young children, can make direct ureteral access difficult. In cases of access failure, ureteral stenting provides passive ureteral dilation and can increase likelihood of successful access in the future. However, routine preprocedural stenting is not recommended.15
Surgical Technique
Following induction of general anesthesia and administration of guideline-directed antibiotic prophylaxis, the patient is positioned into dorsal lithotomy as discussed previously. Isotonic saline irrigation fluid warmed to body temperature is used, given the risk of absorption and hyponatremia.11 A cystoscope is used to gain ureteral access, and a retrograde pyelogram is performed to define collecting system anatomy and determine stone location. A safety guide wire (0.025–0.038 inch) is placed. A working guide wire can also be placed at this time, which allows passage of a ureteral access sheath or flexible ureteroscope. The ureteroscope is introduced into the ureteral orifice following hydrodilation using a hand irrigation pump and is advanced to the stone.21 If the ureteroscope does not pass, a ureteral stent is placed to allow passive ureteral dilation for 2–3 weeks prior to repeating ureteroscopy. Alternatively, active ureteral dilation with an 8–10 Fr dual lumen catheter or coaxial dilator may be employed, but balloon dilation of the ureteral orifice should be avoided due to risk of ureteral stricture.22 If the stone is too large for en bloc basketing, a Holmium:YAG laser is used to treat the stone using fragmentation (high energy, low frequency) or dusting (low energy, high frequency) settings. Large stone fragments are basket extracted and sent for stone analysis and smaller fragments (< 1mm) are left in situ to pass spontaneously. Ureteral access sheaths (9.5–14 Fr) should be used for large proximal ureteral or renal stones, as use is shown to decrease intrarenal pressures, facilitate multiple scope passes and protect the ureter from trauma.23 However, use of a ureteral access sheath is not always feasible due to the small caliber of pediatric ureters. Following completion of lithotripsy, a ureteral stent may be placed. The decision to place a ureteral stent is based on operative time, stone burden, ureteral edema and trauma during the procedure. In select cases, ureteral stents are placed with an externalized string allowing removal without a second procedure. Use of x-ray during the procedure should follow the ALARA concept (as low as reasonably achievable) as children with nephrolithiasis often require more imaging in the future.24 Use of intermittent, low dose x-ray with maximal skin to target distance, narrow image window and avoidance of magnification can reduce procedural radiation exposure.25
Complications
Contemporary series report overall complication rates of ureteroscopy ranging from 0-14% in pediatric patients and include urinary tract infection, hematuria, renal colic, post-operative hydronephrosis and ureteral injury.18,26,27,28 More severe complications (> Clavien 3) are rare, with complete ureteral avulsion and sepsis occurring in <1% of patients.29 Younger age may be associated with an increased risk of complications following ureteroscopic procedures. A systematic review of 10 studies (1,377 procedures) demonstrated a higher complication rate in children < 6 years (24%) compared to children > 6 years (7.1%).28
Postoperative Care and Follow Up
Ureteroscopy is an outpatient surgical procedure in most cases. Postoperative pain is treated with nonsteroidal anti-inflammatories and use of opioid analgesics is limited. A recent double blinded, randomized control trial in adult patients demonstrated 10mg of ketorolac to be noninferior to 5mg of oxycodone for treatment of pain after ureteroscopy.30 Patients with ureteral stents are prescribed anticholinergics and alpha blockers, as use may reduce ureteral stent associated symptoms.31,32 Silent ureteral obstruction following ureteroscopy occurs in up to 3% of patients. Therefore, it is our practice to perform a renal ultrasound 4 weeks after ureteroscopy in all patients.33
Nephroscopy
Indications
Percutaneous nephroscopy with nephrolithotomy is the mainstay of treatment for large renal stones and stones in kidneys with anatomic anomalies that preclude shock wave lithotripsy or retrograde intrarenal surgery. Historically, concerns existed that using adult sized instruments and sheaths (24–30 Fr) in pediatric kidneys would lead to parenchymal damage, and increase the risk of short and long-term complications.19 However, with improvement in technique and miniaturization of nephroscopes, percutaneous nephrolithotomy (PCNL) is regarded as safe and effective in pediatric patients.34 The 2016 AUA/Endourology Society Guidelines on the Surgical Management of Stones recommend PCNL as a treatment option for total renal stone burden of >20mm.15 Additionally, PCNL may be used to treat stones in calyceal diverticula, other anatomically complex kidneys, or when retrograde access is not feasible as with urinary diversion.
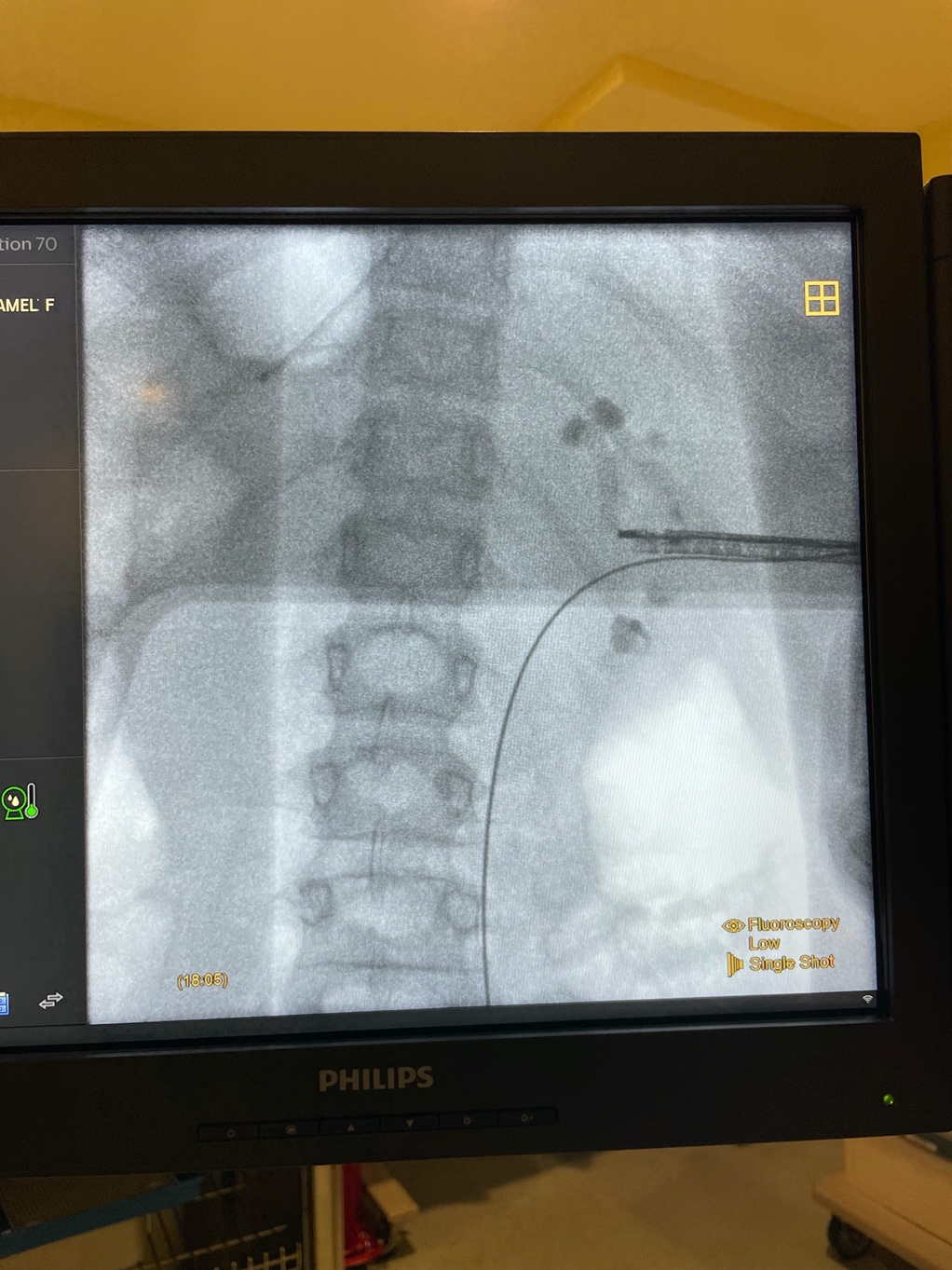
Figure 5 Rigid Nephroscopy. X-ray imaging during relook PCNL for large renal stones using rigid nephroscope with mid-renal access.
Equipment
Rigid nephroscopes allow visualization of the renal pelvis and calyces, are available in fiberoptic and digital models, and come in a variety of sizes (4.8–24 Fr).16 The fiberoptic rod-lens model requires an external camera and light source, while the digital model incorporates the endoscope, light source, and camera into the device.35 A working channel allows introduction of instruments and inflow and outflow ports allow simultaneous filling and emptying of the collecting system.
Intracorporal lithotripters include both ultrasonic, pneumatic and combined models.35 An incorporated large caliber suction removes stone fragments and dust during lithotripsy.
Standard PCNL involves use of a 24–30 Fr Amplatz sheath and adult-sized instruments. Given concern that the use of adult-sized instruments may increase complication rates in pediatric PCNL, techniques using smaller instruments were developed. Mini-PCNL describes use of nephrostomy tracts 15–24 Fr, ultra-mini PCNL with tracts 11–15 Fr and micro-PCNL with tracts < 10 Fr.16 Procedures should be performed in an operating room with experienced personnel, fluoroscopy and appropriate equipment in a variety of sizes. Instruments required for PCNL include rigid and flexible nephroscopes, balloon or Amplatz dilators, access sheaths, Holmium:YAG laser, pneumatic or ultrasonic lithotripter, guide wires, ureteral catheters, dual lumen catheters and baskets.
Preoperative Considerations
All patients considered for PCNL should undergo a low dose CT scan of the abdomen and pelvis to determine feasibility and assess total renal stone burden.15 In addition, cross sectional imaging allows determination of optimal calyx for access, proximity to surrounding organs (colon, pleura, ribs), and alterations in renal anatomy.
A preoperative urine culture should be performed and all patients with a urinary tract infection treated with culture specific antibiotics prior to the procedure. It is important to note that a lower urinary tract culture may not be reflective of stone or urine cultures from the upper tract. The 2019 AUA Best Practice Statement on Urological Procedures and Antimicrobial Prophylaxis recommends the following antibiotic options prior to PCNL based on high quality literature in adult patients: 1st/2nd generation cephalosporin, aminoglycoside and metronidazole, aztreonam and metronidazole, aminoglycoside and clindamycin or aztreonam and clindamycin.12 Literature regarding preoperative antibiotic prophylaxis prior to PCNL in pediatric patients is limited. A retrospective review of 830 children age < 12 years examined the rate of febrile urinary tract infection after PCNL. First, second and third generation cephalosporins were found to be equally efficacious for prophylaxis in children < 12 undergoing PCNL.36 It is our practice to use cefazolin and gentamicin as antibiotic prophylaxis prior to PCNL.
Surgical Considerations
Following induction of general anesthesia and administration of intravenous antibiotics, the patient is positioned prone over gel rolls. Percutaneous access into the desired calyx is performed using fluoroscopic or ultrasound guidance. Alternatively, interventional radiology can obtain CT guided percutaneous access in cases of aberrant anatomy or spinal dysraphism. Multiple accesses may be needed for treatment of partial or complete staghorn calculi. A working and safety guide wire are placed, skin is incised and the tract is dilated with a balloon or Amplatz dilators, based on surgeon preference. Choice of dilator and sheath size is based on patient age, body habitus, anatomy, stone burden, and available nephroscope size. Note that pediatric kidneys are more mobile than adult kidneys and may push away when dilating or advancing the sheath.37 The sheath is then placed over the dilator into the collecting system. Trimming the sheath may improve maneuverability. Warmed isotonic fluid is used given propensity for hypothermia and hypervolemia. The rigid nephroscope is advanced to the stone and fragmentation commences, using either a Holmium:YAG laser or lithotripter. Stone fragments are extracted and sent for stone analysis and culture. A flexible cystoscope or ureteroscope is used to perform flexible nephroscopy and residual stone fragments are extracted. Following clearance of stone burden, a percutaneous nephrostomy tube, ureteral stent or both may be placed. This decision is based on surgical complexity, residual stone burden, need for relook procedure and surgeon preference.38 We recommend limiting operative time to 90 minutes as prolonged operative times increase the risk of postoperative febrile UTI.36
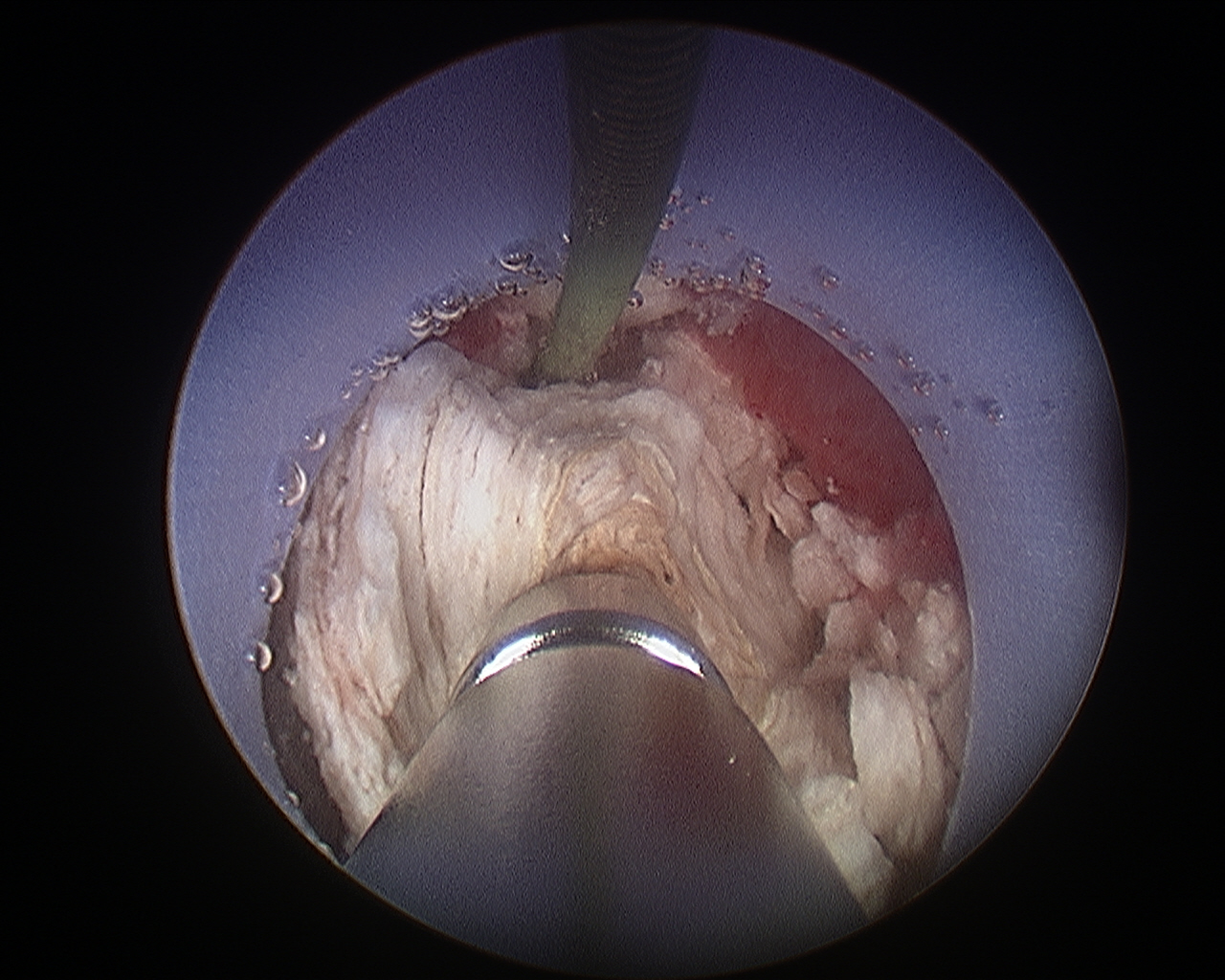
Figure 6 Percutaneous Nephrolithtomy. Ultrasonic lithotripsy of a large renal stone during percutaneous nephrolithotomy.
Complications
Contemporary series report complication rates following pediatric PCNL of 11–40%.34,39 The most common complications are febrile urinary tract infection and bleeding requiring transfusion.34,39 Severe complications such as massive bleeding, urine leak requiring drainage, visceral injury (colon, pleura), renal pelvis injury and hydrothorax are rare, with 84% of complications < Clavien grade III.34,39 Stone size, case complexity and operative time are associated with complication rates.34,40 Ultra-mini and micro PCNL using smaller access tracts demonstrate lower overall complication rates (11.2%).41
Postoperative Care and Follow Up
Following PCNL, patients are hospitalized for 2–5 days. Perioperative antibiotics are continued for 24 hours after surgery. Laboratory tests are obtained to evaluate for electrolyte abnormalities and/or significant blood loss. Postoperative imaging is obtained to evaluate for residual stone burden and plan additional surgical treatment. If no relook PCNL is planned and gross hematuria has cleared, the nephrostomy tube is removed prior to hospital discharge. Frequently, additional procedures are required to achieve complete stone clearance. Stone free rates following PCNL range from 63–85.4% and increase to 91.7–93.7% with subsequent treatment (re-look PCNL, shock wave lithotripsy, retrograde intrarenal surgery).34,42,43
Conclusions
Technologic advancements in endoscopic equipment have transformed the field of pediatric urology, improving diagnostic and therapeutic potential. Conditions formerly treated with open surgical procedures are now managed endoscopically. As device innovation progresses at an exponential rate, so too should high quality prospective studies to elucidate the role of this new technology in the pediatric urologist’s armamentarium.
Key Points
- Miniturization of adult endoscopic instruments and improvements in technology led to the development of pediatric cystoscopes, ureteroscopes and nephroscopes
- Choice of endoscope type and size should be tailored to the child’s age, size, body habitus and unique anatomy
- Routine preprocedural antibiotic prophylaxis is not recommended for uncomplicated cystoscopy, but is indicated for upper urinary tract instrumentation
- When performing pediatric endoscopy, we should follow the ALARA concept to limit procedural radiation exposure
- Complete stone clearance with ureteroscopy or PCNL may require multiple procedures given the small caliber of the pediatric collecting system and ureter
Resources for Patients
- https://www.urologyhealth.org/healthy-living/urologyhealth-extra/magazine-archives/summer-2020/kidney-stones-in-children
- https://pedsnet.org/pkids/
Suggested Readings
- Tekgül S, Stein R, Bogaert G, Nijman RJM, Quaedackers J, Hoen L ’t, et al.. European Association of Urology and European Society for Paediatric Urology Guidelines on Paediatric Urinary Stone Disease. Eur Urol Focus 2021; 8 (3): 833–839. DOI: 10.1016/j.euf.2021.05.006.
- Kokorowski PJ, Chow JS, K S. Prospective Measurement of Patient Exposure to Radiation During Pediatric Ureteroscopy. Yearbook of Urology 2012; 2012 (4): 224–225. DOI: 10.1016/j.yuro.2012.07.025.
- Duty B, Conlin M. Principles of Urologic Endoscopy. In: Partin A, editor. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021. DOI: 10.1016/s0025-6196(12)60891-x.
References
- REUTER MATTHIASA, REUTER HANSJ. The Development Of The Cystoscope. J Urol 1998; 59 (3): 638–640. DOI: 10.1016/S0022-5347(01)63691-7.
- Shah J. Endoscopy through the ages. BJU Int 2002; 89 (7): 645–652. DOI: 10.1046/j.1464-410x.2002.02726.x.
- Samplaski MK, Jones JS. Two centuries of cystoscopy: the development of imaging, instrumentation and synergistic technologies. BJU Int 2009; 103 (2): 154–158. DOI: 10.1111/j.1464-410x.2008.08244.x.
- Talwar HS. The journey of the “Lichtleiter”, the first ever cystoscope: An ode to Philipp Bozzini and his great invention. Eur Urol 1969; 81: S751. DOI: 10.1016/s0302-2838(22)00581-4.
- Nicholson P. Problems encountered by early endoscopists. Urology 1982; 19 (1): 114–119. DOI: 10.1016/0090-4295(82)90065-6.
- Herr HW. Max Nitze, the Cystoscope and Urology. J Urol 2006; 176 (4): 1313–1316. DOI: 10.1016/j.juro.2006.06.085.
- Sasian J. Harold H. Hopkins. Introduction to Aberrations in Optical Imaging Systems 1998; 2 (1): xvii–xviii. DOI: 10.1017/cbo9780511795183.
- Berci G, Paz-Partlow M. Electronic imaging in endoscopy. Surg Endosc 1988; 2 (4): 227–233. DOI: 10.1007/bf00705327.
- Gobbi D, Midrio P, Gamba P. Instrumentation for minimally invasive surgery in pediatric urology. Transl Pediatr 2015; 5 (4): 186–204. DOI: 10.21037/tp.2016.10.07.
- Osborn NK, Baron TH. The history of the “French” gauge. Gastrointest Endosc 2006; 63 (3): 461–462. DOI: 10.1016/j.gie.2005.11.019.
- Duty B, Conlin M. Principles of Urologic Endoscopy. In: Partin A, editor. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021. DOI: 10.1016/s0025-6196(12)60891-x.
- Lightner DJ, Wymer K, Sanchez J, Kavoussi L. Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis. J Urol 2020; 203 (2): 351–356. DOI: 10.1097/ju.0000000000000509.
- TURAN HALE, BALCI UGUR, ERDINC FSEBNEM, TULEK NECLA, GERMIYANOGLU CANKON. Bacteriuria, pyuria and bacteremia frequency following outpatient cystoscopy. Int J Urol 2006; 13 (1): 25–28. DOI: 10.1111/j.1442-2042.2006.01219.x.
- Salerno A, Nappo SG, Matarazzo E, De Dominicis M, Caione P. Treatment of pediatric renal stones in a Western country: A changing pattern. J Pediatr Surg 2013; 48 (4): 835–839. DOI: 10.1016/j.jpedsurg.2012.09.058.
- Assimos D, Krambeck A, NL M. Faculty Opinions recommendation of Surgical management of stones: american urological association/endourological society guideline, PART I. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2016; 96 (4): 161–169. DOI: 10.3410/f.726388210.793519339.
- Silay MS. Recent Advances in the Surgical Treatment of Pediatric Stone Disease Management. European Urology Supplements 2017; 16 (8): 182–188. DOI: 10.1016/j.eursup.2017.07.002.
- Payne DA KJFX. Rigid and flexible ureteroscopes: technical features. In: Smith AD, Badlani GH, Preminger GM, Kavoussi LR, editors. Smith’s Textbook of Endourology. 3rd ed. West Sussex, UK: Wiley-Blackwell;2012:365-87; . DOI: 10.1002/9781444345148.ch34.
- Kucukdurmaz F, Efe E, Sahinkanat T, Amasyalı AS, Resim S. Ureteroscopy With Holmium:Yag Laser Lithotripsy for Ureteral Stones in Preschool Children: Analysis of the Factors Affecting the Complications and Success. Urology 2018; 111: 162–167. DOI: 10.1016/j.urology.2017.09.006.
- Tasian GE, Copelovitch LA. Management of pediatric kidney stone disease. In: Partin A, editor. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021. DOI: 10.1007/s11934-007-0067-8.
- Davis NF, Quinlan MR, Browne C, Bhatt NR, Manecksha RP, D’Arcy FT, et al.. Single-use flexible ureteropyeloscopy: a systematic review. World J Urol 2018; 36 (4): 529–536. DOI: 10.1007/s00345-017-2131-4.
- Soygur T, Zumrutbas AE, Gulpinar O, Suer E, Arikan N. Hydrodilation of the Ureteral Orifice in Children Renders Ureteroscopic Access Possible Without any Further Active Dilation. J Urol 2006; 176 (1): 285–287. DOI: 10.1016/s0022-5347(06)00580-5.
- MINEVICH EUGENE, DeFOOR WILLIAM, REDDY PRAMOD, NISHINAKA KAZUYUKI, WACKSMAN JEFFREY, SHELDON CURTIS, et al.. Ureteroscopy Is Safe And Effective In Prepubertal Children. J Urol 2005; 174 (1): 276–279. DOI: 10.1097/01.ju.0000161212.69078.e6.
- Auge BK, Pietrow PK, Lallas CD, Raj GV, Santa-Cruz RW, Preminger GM. Ureteral Access Sheath Provides Protection against Elevated Renal Pressures during Routine Flexible Ureteroscopic Stone Manipulation. J Endourol 2004; 18 (1): 33–36. DOI: 10.1089/089277904322836631.
- Kuhns LR, Oliver WJ, Christodoulou E, Goodsitt MM. The Predicted Increased Cancer Risk Associated With a Single Computed Tomography Examination for Calculus Detection in Pediatric Patients Compared With the Natural Cancer Incidence. Pediatr Emerg Care 2011; 27 (4): 345–350. DOI: 10.1097/pec.0b013e3182132016.
- Kokorowski PJ, Chow JS, K S. Prospective Measurement of Patient Exposure to Radiation During Pediatric Ureteroscopy. Yearbook of Urology 2012; 2012 (4): 224–225. DOI: 10.1016/j.yuro.2012.07.025.
- Tolga-Gulpinar M, Resorlu B, Atis G, Tepeler A, Ozyuvali E, Oztuna D, et al.. Safety and efficacy of retrograde intrarenal surgery in patients of different age groups. Actas Urol Esp (Engl Ed) 2015; 39 (6): 354–359. DOI: 10.1016/j.acuroe.2015.05.005.
- Dogan HS, Onal B, N S. Factors Affecting Complication Rates of Ureteroscopic Lithotripsy in Children: Results of Multi-Institutional Retrospective Analysis by Pediatric Stone Disease Study Group of Turkish Pediatric Urology Society. J Urol 2011; 186 (3): 1035–1040. DOI: 10.1016/j.juro.2011.04.097.
- Ishii H, Griffin S, Somani BK. Flexible ureteroscopy and lasertripsy (FURSL) for paediatric renal calculi: Results from a systematic review. J Pediatr Urol 2014; 11 (3): 164. DOI: 10.1016/j.jpurol.2015.01.010.
- De Coninck V, Keller EX, Somani B, Giusti G, Proietti S, Rodriguez-Socarras M, et al.. Complications of ureteroscopy: a complete overview. World J Urol 2020; 38 (9): 2147–2166. DOI: 10.1007/s00345-019-03012-1.
- Fedrigon D, Faris A, N K. SKOPE–Study of Ketorolac vs Opioid for Pain after Endoscopy: A Double-Blinded Randomized Control Trial in Patients Undergoing Ureteroscopy. Reply. J Urol 2021; 206 (6): 1529–1530. DOI: 10.1097/ju.0000000000002194.
- Zhou L, Cai X, Li H, Wang K-jie. Effects of \ensuremathα-Blockers, Antimuscarinics, or Combination Therapy in Relieving Ureteral Stent-Related Symptoms: A Meta-Analysis. J Endourol 2015; 29 (6): 650–656. DOI: 10.1089/end.2014.0715.
- Sivalingam S, Streeper NM, Sehgal PD, Sninsky BC, Best SL, Nakada SY. Does Combination Therapy with Tamsulosin and Tolterodine Improve Ureteral Stent Discomfort Compared with Tamsulosin Alone? A Double-Blind, Randomized, Controlled Trial. J Urol 2016; 195 (2): 385–390. DOI: 10.1016/j.juro.2015.08.104.
- Weizer AZ, Auge BK, Silverstein AD, Delvecchio FC, Brizuela RM, Dahm P, et al.. Routine Postoperative Imaging is Important After Ureteroscopic Stone Manipulation. J Urol 2002; 68 (2): 46–50. DOI: 10.1016/S0022-5347(05)64829-X.
- Goyal NK, Goel A, Sankhwar SN, Singh V, Singh BP, Sinha RJ, et al.. A critical appraisal of complications of percutaneous nephrolithotomy in paediatric patients using adult instruments. BJU Int 2014; 113 (5): 801–810. DOI: 10.1111/bju.12506.
- Canales BK, Pugh JW. New instrumentation in percutaneous nephrolithotomy. Indian J Urol 2010; 26 (3): 389. DOI: 10.4103/0970-1591.70579.
- Kaygısız O, Satar N, Güneş A, Doğan HS, Erözenci A, Özden E, et al.. Factors predicting postoperative febrile urinary tract infection following percutaneous nephrolithotomy in prepubertal children. J Pediatr Urol 2018; 14 (5): 448.e1–448.e7. DOI: 10.1016/j.jpurol.2018.04.010.
- Tekgül S, Stein R, Bogaert G, Nijman RJM, Quaedackers J, Hoen L ’t, et al.. European Association of Urology and European Society for Paediatric Urology Guidelines on Paediatric Urinary Stone Disease. Eur Urol Focus 2021; 8 (3): 833–839. DOI: 10.1016/j.euf.2021.05.006.
- Aghamir SMK, Salavati A, Aloosh M, Farahmand H, Meysamie A, Pourmand G. Feasibility of Totally Tubeless Percutaneous Nephrolithotomy Under the Age of 14 Years: A Randomized Clinical Trial. J Endourol 2012; 26 (6): 621–624. DOI: 10.1089/end.2011.0547.
- Hosseini MM, Irani D, Altofeyli A, Eslahi A, Basiratnia M, Haghpanah A, et al.. Outcome of Mini-Percutaneous Nephrolithotomy in Patients Under the Age of 18: An Experience With 112 Cases. Front Surg 2021; 8 (613812). DOI: 10.3389/fsurg.2021.613812.
- Nouralizadeh A, Basiri A, Javaherforooshzadeh A, Soltani MH, Tajali F. Experience of percutaneous nephrolithotomy using adult-size instruments in children less than 5 years old. J Pediatr Urol 2009; 5 (5): 351–354. DOI: 10.1016/j.jpurol.2008.12.009.
- Çitamak B, Dogan HS, Ceylan T, Hazir B, Bilen CY, Sahin A, et al.. A new simple scoring system for prediction of success and complication rates in pediatric percutaneous nephrolithotomy: stone-kidney size score. J Pediatr Urol 2019; 15 (1): 67.e1–67.e6. DOI: 10.1016/j.jpurol.2018.09.019.
- Baydilli N, Tosun H, Akınsal EC, Gölbaşı A, Yel S, Demirci D. Effectiveness and complications of mini-percutaneous nephrolithotomy in children: one center experience with 232 kidney units. Turk J Urol 2019; 46 (1): 69–75. DOI: 10.5152/tud.2019.19158.
- Jones P, Bennett G, Aboumarzouk OM, Griffin S, Somani BK. Role of Minimally Invasive Percutaneous Nephrolithotomy Techniques–Micro and Ultra-Mini PCNL (<15F) in the Pediatric Population: A Systematic Review. J Endourol 2017; 31 (9): 816–824. DOI: 10.1089/end.2017.0136.
- Xue W, Pacik D, Boellaard W, Breda A, Botoca M, Rassweiler J, et al.. Management of Single Large Nonstaghorn Renal Stones in the CROES PCNL Global Study. J Urol 2012; 187 (4): 1293–1297. DOI: 10.1016/j.juro.2011.11.113.
- Unsal A, Resorlu B, Kara C, Bozkurt OF, Ozyuvali E. Safety and Efficacy of Percutaneous Nephrolithotomy in Infants, Preschool Age, and Older Children With Different Sizes of Instruments. Urology 2010; 76 (1): 247–252. DOI: 10.1016/j.urology.2009.08.087.
Ultima atualização: 2023-05-01 09:16