15: Duplex, Migration, and Fusion Anomalies
Este capítulo durará aproximadamente 26 minutos para leer.
Introduction
Duplication, migration, and fusion anomalies of the urinary tract are common and lead to various structural and functional abnormalities. Anatomic sequelae of ureteral duplication include ectopic ureter, ureterocele, and vesicoureteral reflux. These may manifest clinically as urinary tract obstruction, infection, incontinence, and renal dysplasia. Common migration and fusion anomalies include renal ectopia and horseshoe kidney, which are occasionally associated with pathology including renal dysplasia, vesicoureteral reflux, obstruction, and stone disease. Severity varies widely, and treatment ranges from observation to major surgical reconstruction. Treatment, when indicated, is primarily surgical and is aimed at preservation of renal function, minimizing morbidity from urinary tract infection, correction of urinary incontinence, and relief of symptoms associated with urinary tract obstruction.
Embryology
Familiarity with the normal embryology of renal development is critical to understanding duplication, migration and fusion anomalies. Two embryologic structures give rise to the mature human kidney and ureters; the ureteric bud, which develops into the collecting system and ureters, and the metanephric mesenchyme, which develops into the nephrons and renal parenchyma. The ureteric bud originates as an epithelial outpouching off the distal mesonephric (Wolffian) duct. Normally, there is a single ureteric bud on each side which give rise to bilateral single ureters. The ureteric bud grows into the metanephric mesenchyme and a process of reciprocal induction causes nephrogenesis and ureteral branching. The renal unit with its branched collecting system (metanephros) migrates cranially and rotates 90 degrees such that the renal hilum is oriented medially. The ureter grows caudally to merge with the urogenital sinus, which becomes the bladder. Once incorporated into the urogenital sinus, the ureteral orifice migrates craniolaterally from the bladder neck to the orthotopic position on the trigonal ridge.1
Duplication anomalies are thought to stem from supernumerary ureteral buds or early branching. Migration anomalies result from abnormal ascent or rotation. If the kidneys come into contact with each other they may fuse, resulting in a horseshoe kidney or crossed-fused renal ectopia.2
Epidemiology and Pathophysiology
Table 1 Common clinical pathologies in renal duplication.
| Anomaly | Potential problems |
|---|---|
| Incomplete duplication | Lower pole UPJ obstruction Ureteroureteral reflux (theoretical) |
| Ectopic ureter | Upper pole obstruction Urinary incontinence Pyelonephritis Epididymitis |
| Ureterocele | Upper pole obstruction Lower pole obstruction Bladder outlet obstruction Dysfunctional voiding Urinary incontinence due to sphincter disruption Pyelonephritis |
| Vesicoureteral reflux | Reflux nephropathy Pyelonephritis |
Duplication
Renal duplication anomalies are the most common congenital urinary tract anomaly with an incidence of 0.8–5% noted on autopsy studies.3,4 A duplex kidney refers to a kidney with two moieties, upper and lower, each with an independent pyelocaliceal drainage system. Bilateral duplication occurs in 16–40% of cases, and duplication is detected more commonly in females.3,5 Duplication anomalies can be hereditary, with an incidence of 12–30% in affected families.6,7
Duplication may be incomplete (partial) or complete. In incomplete duplication, the two collecting systems may join at any point before entering the bladder. This occurs when a single ureteral bud branches prior to reaching the metanephric mesenchyme.3 Most ureteral duplications are incomplete and clinically insignificant. Partial duplication ranges from a bifid renal pelvis to a Y-type duplication with the ureters joining at any point prior to their insertion into the bladder. One problem encountered with incomplete duplication is obstruction of the ureteropelvic junction (UPJ), which occurs almost exclusively at the lower pole.8,9 The intrarenal architecture in duplicated systems may explain the predilection of UPJ obstructions for the lower pole. Typically, the upper-pole ureter drains a single infundibulum without a true pelvis. The lower pole, which comprises two-thirds of the parenchyma, usually contains at least two major calices and a true renal pelvis.9
Clinically-significant pathology more commonly arises from complete duplication. In complete duplication, independent ureters drain the upper and lower renal moieties and empty separately into the genitourinary tract. This occurs when two ureteral buds branch off the mesonephric duct. Each bud or branch induces maturation of an overlying renal unit. Distally, the lower ureter will be incorporated into the urogenital sinus first and will migrate further craniolaterally. The upper ureter is incorporated later or not at all and is thus medially and caudally displaced in the bladder or, if it never reaches the urogenital sinus, is ectopically located along the mesonephric duct derivatives. The craniolateral ureteral orifice is thus reliably associated with the lower renal moiety and the caudomedial ureteral orifice with the upper renal moiety, an embryologic relationship known as the Weigert-Meyer law.10,11 Rarely, additional ureteral buds or early branching may lead to ureteral triplication (Figure 1).
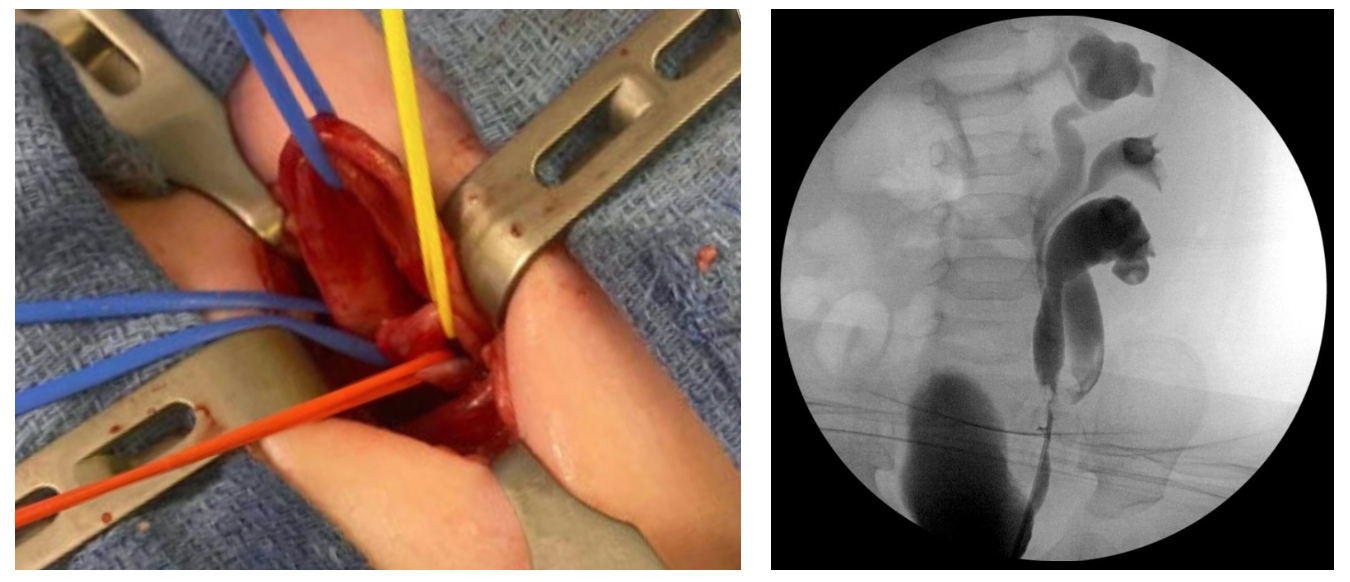
Figure 1 Ureteral triplication. A: Intraoperative photo during upper to lower ureteroureterostomy. The proximal blue vessel loop surrounds the upper moiety ureter, which is ectopic to the bladder neck with obstruction. The red and yellow vessel loops surround the mid and lower moiety ureters, which are incompletely duplicated. B: Retrograde ureteropyelogram after ureteroureterostomy.
Ectopic Ureter
An ectopic ureter typically inserts caudal to the normal location on the trigonal ridge, along the pathway of the developing mesonephric system. In boys, ectopic ureters may open into bladder neck, posterior urethra (most common), or mesonephric duct derivatives: epididymis, seminal vesicle, or vas deferens.12 Notably, these locations are proximal to the urethral sphincter complex and thus never result in incontinence. By contrast, ectopic ureters in girls can terminate distal to the external sphincter and cause continuous urinary incontinence. The most common terminal sites of ectopic ureters in girls are the bladder neck and upper urethra (1/3), vaginal vestibule (1/3), vagina (1/4), and cervix, uterus, or rectum (rare).12,13 Approximately 75–90% of ectopic ureters are associated with the upper moiety of a duplex kidney.14,15
Problems that result from ureteral ectopia include ureteral obstruction with resultant renal dysplasia and/or risk for infection, continuous urinary incontinence in girls, and occasionally epididymitis from urine reflux into the vas deferens in boys.
Ureterocele
A ureterocele is a cystic dilatation of the terminal part of the ureter. The embryology is not entirely understood. One theory posits that ureteroceles result from persistence of Chwalle’s membrane, the primitive ureteral membrane that separates the ureteric bud from the developing urogenital sinus. Alternatively, some think that a ureterocele results from abnormal induction of bladder trigonal musculature.16,17
Various classification systems have been proposed based on the location of the ureteral opening, characteristics of the orifice, or association with single or duplex collecting systems. The classification adopted by the Section on Urology of the American Academy of Pediatrics is perhaps the simplest and categorizes ureteroceles based on the location of the dilated ureteral tissue.18 Ureteroceles are classified as either intravesical (orthotopic) or extravesical (ectopic). Intravesical ureteroceles are contained entirely within the bladder (Figure 2). Ureteroceles extending beyond the bladder neck are classified as extravesical. Occasionally, distinguishing the two can be difficult. For instance, an intravesical ureterocele may prolapse through the bladder neck and an extravesical ureterocele can have an intravesical ureteral orifice (Figure 3). Extravesical ureteroceles with submucosal extension beyond the bladder neck are sometimes called cecoureteroceles.
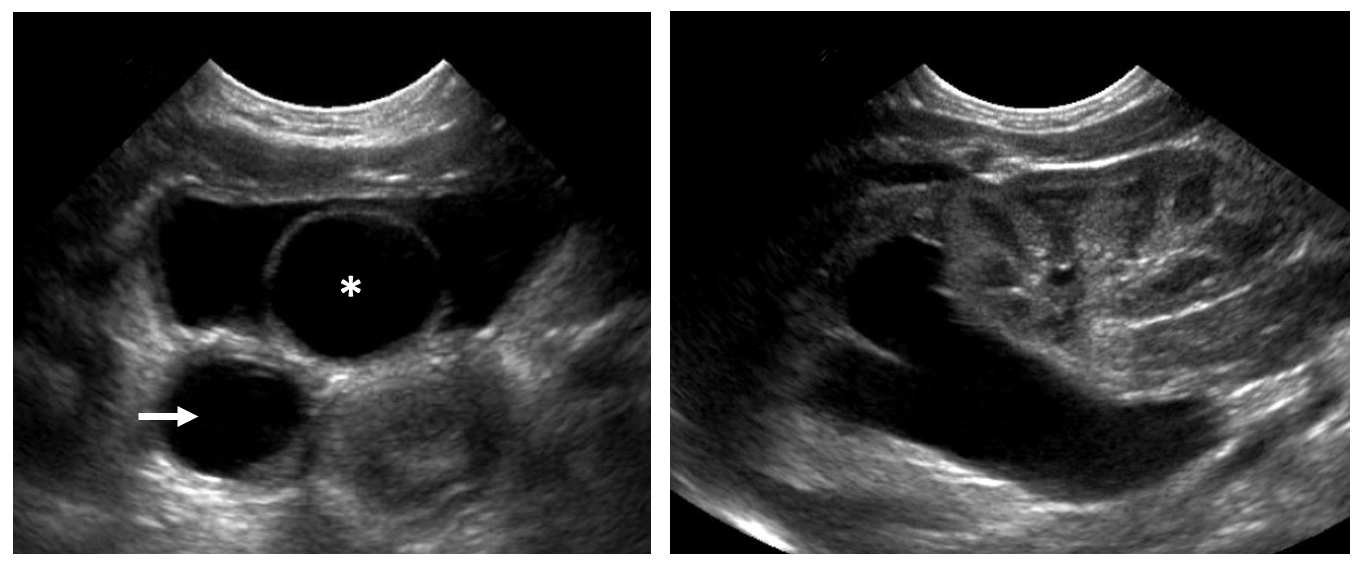
Figure 2 Intravesical ureterocele. A: An intravesical ureterocele with its characteristic thin-walled cystic appearance on ultrasound (asterisk). Marked ureteral dilation is noted (arrow). B: The upper moiety associated with the ureterocele is hydronephrotic with renal parenchymal thinning. The lower moiety appears healthy.
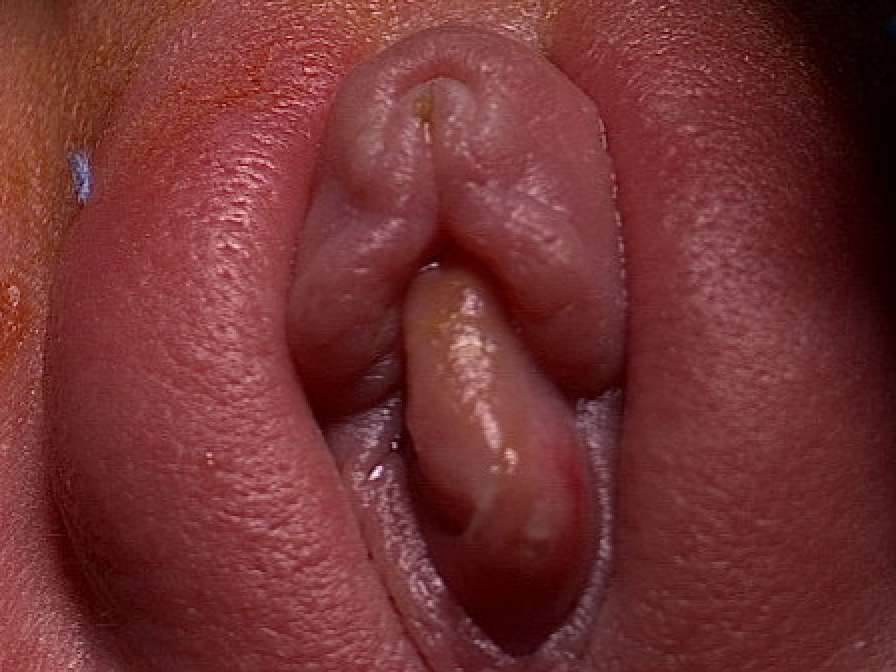
Figure 3 Ureterocele prolapse in a female infant.
In duplex systems, ureteroceles are nearly always associated with the upper pole and 60% are extravesical.16 Problems include obstruction of the upper and possibly the lower pole, which in the setting of urinary tract infection can present as florid sepsis. Obstruction is often associated with a dysplastic upper pole.19 Additionally, ureteroceles that prolapse can obstruct the bladder outlet. Large ureteroceles are associated with poor underlying bladder musculature, and ureteroceles that extend beyond the bladder neck can destroy the female continence mechanism.20,21
Vesicoureteral Reflux
The combination of ureteral duplication and vesicoureteral reflux is common. Reflux classically occurs into the lower pole ureter which has a laterally displaced ureteral orifice and shortened submucosal tunnel as predicted by the Weigert-Meyer law.22 The upper pole ureter, with its more medial and caudal orifice, has a longer submucosal tunnel and reflux is less likely. When reflux into upper and lower pole ureters occurs, both orifices tend to be laterally displaced or the duplication is partial. Reflux into the upper pole ureter may also occur if its orifice is ectopic to the bladder neck or urethra, presumably because it bypasses the trigone and has an inadequate submucosal tunnel. Reflux into such ureters only occurs during voiding, and the tonically closed bladder neck may cause obstruction during the storage phase (Figure 4). As in non-duplicated systems, sequelae of vesicoureteral reflux in duplicated systems include reflux nephropathy and pyelonephritis.
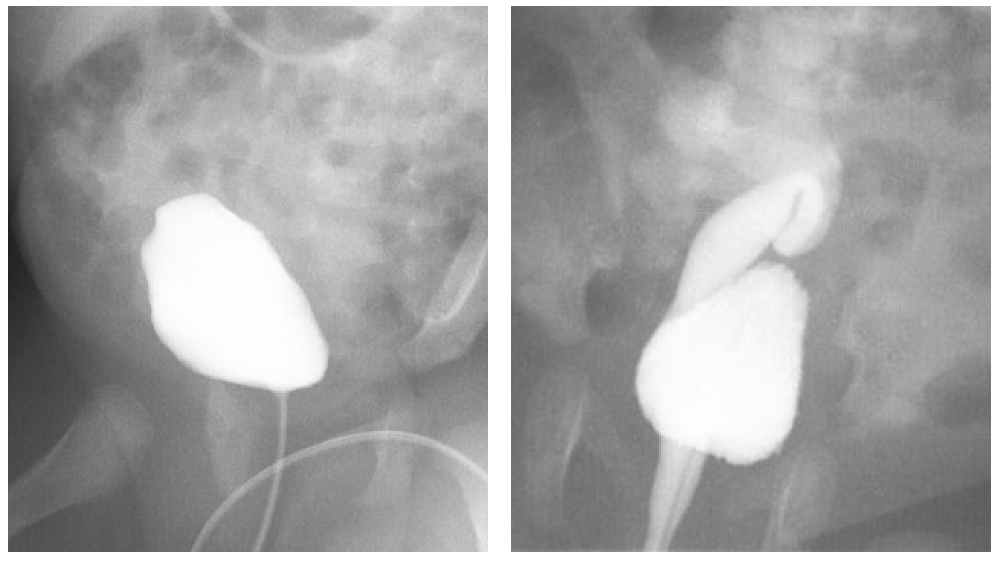
Figure 4 Ectopic ureter inserting at the bladder neck. A: During bladder filling, no vesicoureteral reflux is noted. B: Vesicoureteral reflux becomes apparent during voiding. This kidney is noted to be ectopically located in the pelvis.
Migration and Fusion Anomalies
Renal ectopia refers to a kidney which fails to reach its normal position, and results from abnormal renal migration and/or fusion during embryologic development.23 Ectopic kidneys can be classified as simple, horseshoe or crossed. The reported incidence ranges from 1 in 500 to 1 in 5,000.24,25
Simple Ectopia
Simple renal ectopia refers to a kidney located anywhere along the ipsilateral embryological path of ascent from the pelvis to the renal fossa. Pelvic kidneys fail to ascend past the pelvic brim and are the most common ectopic kidneys, accounting for 60% of all cases.26 In 90% the anomaly is unilateral and with a slight left-sided preponderance.26 Other ectopic kidney positions can occur and lie at some point between the pelvis and the normal position or, rarely, within the thorax.27
Horseshoe Kidney
Horseshoe kidney is the most common renal fusion anomaly, with an incidence between 1:400 and 1:1800.28,29 In 95% of cases the lower poles of the two kidneys are joined by an isthmus of renal tissue, which usually consists of vascularized parenchyma but is sometimes dysplastic or fibrous.29 In about 40% of cases the isthmus lies at the level of L4 where it gets caught beneath the origin of the inferior mesenteric artery during renal ascent. Rarely, the isthmus can lie posterior to the aorta and/or inferior vena cava.30 A small proportion of horseshoe kidneys are fused at their upper poles.31 The presence of the isthmus prevents complete medial rotation of the renal pelves and the ureters must arch anteriorly to pass over the isthmus, which explains the relatively high incidence of ureteropelvic anomalies (20%) associated with horseshoe kidneys (Figure 5).32,33,34
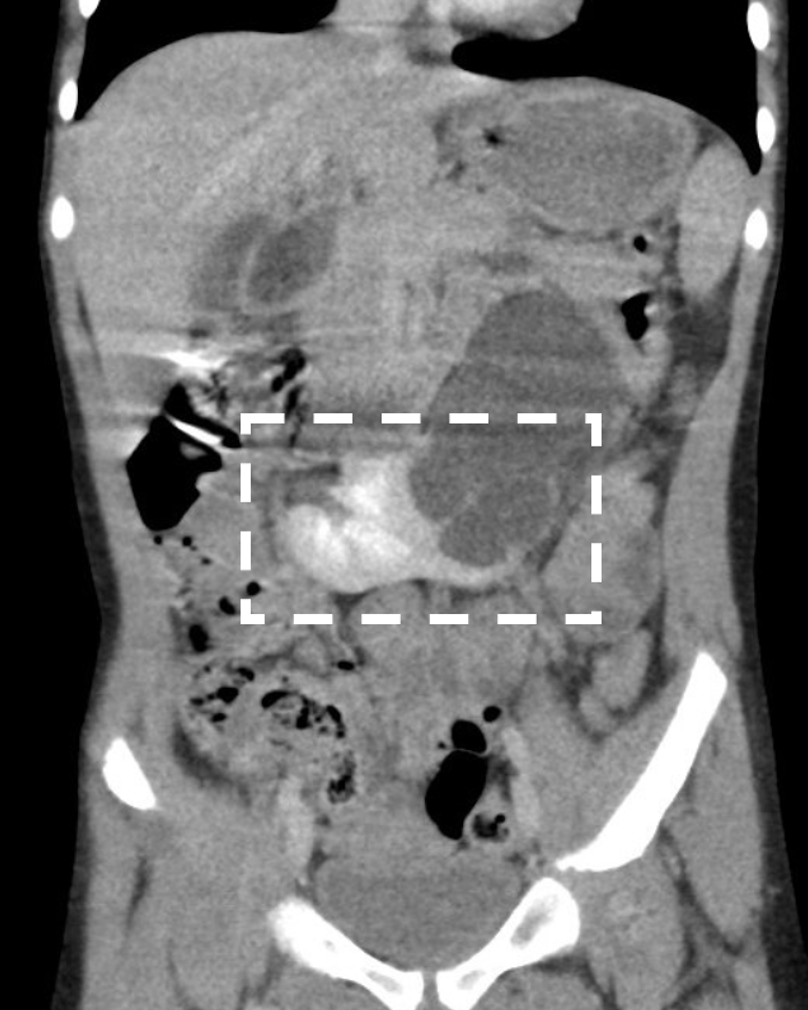
Figure 5 Horseshoe kidney with left ureteropelvic junction obstruction. The isthmus (fused lower poles) is marked by a square. Hydronephrosis is noted on the left side.
Crossed Renal Ectopia
Crossed renal ectopia occurs when one or both kidneys cross the midline during migration. There are four varieties of crossed renal ectopia: crossed fused renal ectopia wherein the ectopic kidney fuses with the contralateral kidney (85% of cases), crossed renal ectopia without fusion, bilateral crossed ectopia, and solitary crossed ectopia. There is a slight male predominance, and crossing from left to right occurs more frequently than from right to left. In crossed fused renal ectopia, the point of fusion is usually between the upper pole of the crossed kidney and the lower pole of the normally positioned kidney (unilateral fused type (Figure 6).35,36,37,38
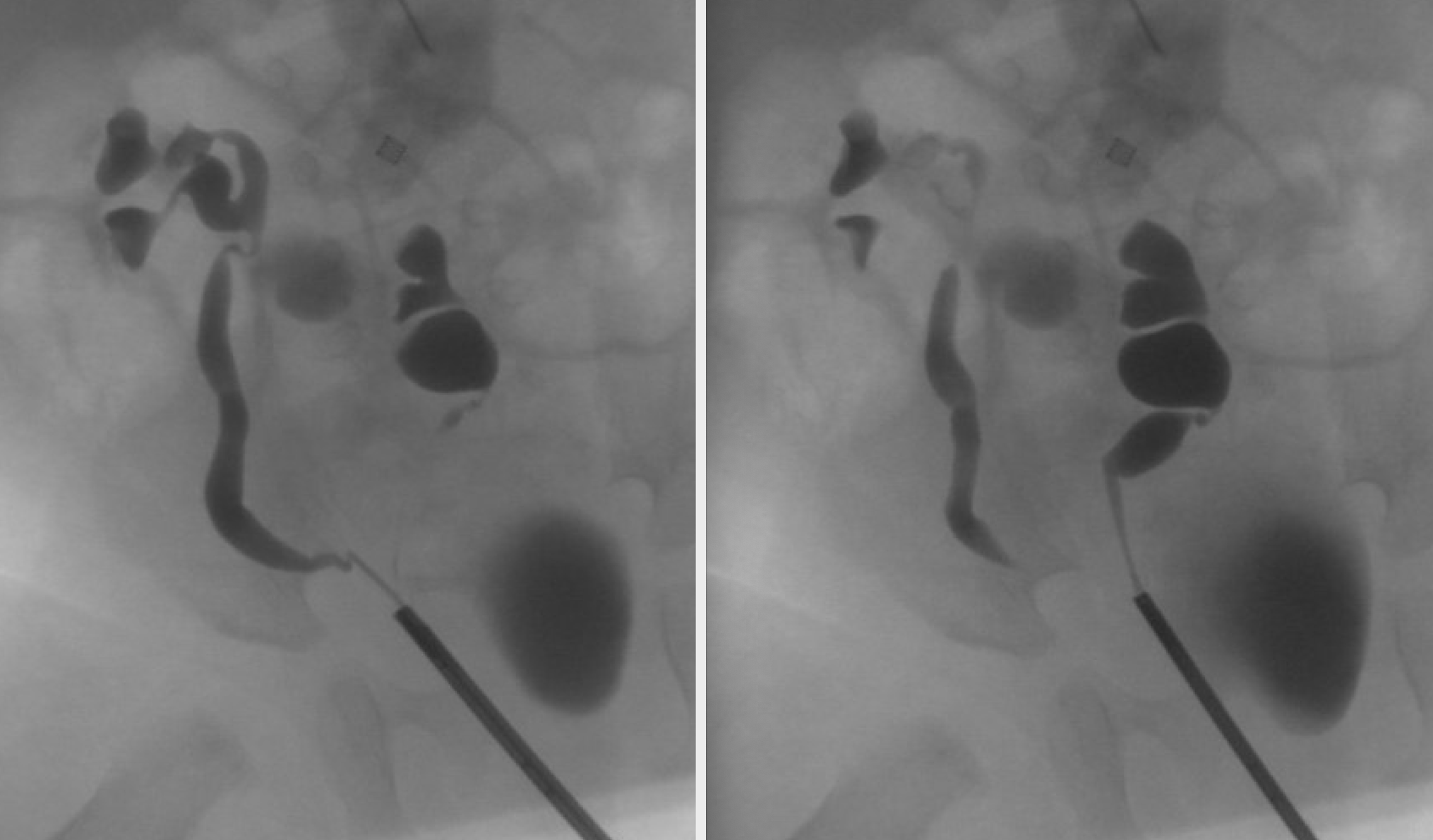
Figure 6 Crossed fused renal ectopia. Retrograde pyelogram demonstrates distinct ureters. The left kidney is located to the right of midline. Its upper pole is fused to the lower pole of the right kidney.
Ectopic kidneys are often asymptomatic. When detected, they are commonly hypoplastic or irregular in shape. Vesicoureteral reflux is the most common associated anomaly, which has been reported in 30–70% of kidneys in children with renal ectopia. Interestingly, in cases of simple renal ectopia, unilateral reflux is frequently seen in the orthotopic kidney.32,33,39 Malrotation and high insertion of the ureter may lead to ureteropelvic junction obstruction, urine stasis, and associated nephrolithiasis.32,33,34 Further, because kidneys derive their vasculature from nearby vessels during embryological ascent, the vascular supply is predictably anomalous in renal ectopia and fusion anomalies. In one study of 90 horseshoe kidneys, 387 arteries were identified, and nearly one fourth of patients had anomalous renal vein anatomy.40
Migration and fusion anomalies are also commonly associated with non-urologic anomalies. Horseshoe kidney, for instance, is present in 14–20% of girls with Turner syndrome.29 Renal ectopia and horseshoe kidneys are frequently seen in children with anorectal malformations and genital anomalies, sometimes as part of the VACTERL syndrome.41 In males, genital anomalies may include hypospadias and cryptorchidism.32 While renal agenesis is the classic genitourinary finding in females with Müllerian anomalies, uterine duplication and vaginal agenesis have also been reported in association migration and fusion anomalies.42,43
Evaluation and Diagnosis
Duplication
Duplication anomalies may be diagnosed antenatally by routine sonogram. Postnatally, duplication is frequently diagnosed upon workup for urinary tract infection (UTI); approximately 8% of children who present with a UTI are found to have duplication.44,45,46 In toilet-trained girls duplication may present with incontinence if an ectopic ureter inserts distal to the urinary sphincter complex.13,47 Children may also present with symptoms of urinary tract obstruction such as intermittent pain or failure to thrive.48 Commonly, duplication presents as an incidental finding on imaging.49
Evaluation of ureteral duplication focuses on the diagnosis, prevention, and management of hydronephrosis, urinary tract infection, renal dysfunction and voiding problems. Incidentally detected duplication in the absence of these findings does not require further investigation. Increasingly, duplication anomalies are being detected antenatally as hydronephrosis or ureteroceles are noted on obstetric sonograms.50 Rarely, bilateral renal dysplasia or significant renal scarring may be present, resulting in impaired renal function. As with all children with renal dysplasia, renal function and electrolyte levels must be monitored, children should be screened for hypertension and proteinuria. Various tests may yield valuable information regarding complete or incomplete ureteral duplication, ectopia, ureteral dilation, ureteroceles, and relative renal function.
Ultrasound
Essentially all patients with renal duplication require an ultrasound of the kidneys and bladder. This will detect upper and/or lower pole hydronephrosis, hydroureter, ureteroceles, and give some information as to the function of each renal unit based on the appearance of the renal parenchyma. Upper moiety hydronephrosis may occur secondary to an obstructed ectopic ureter or ureterocele. Frequently, associated hydroureter is seen (Figure 2) Marked dysplasia is commonly seen secondary to obstructive nephropathy. Normal renal parenchyma without ureteral dilation can suggest incidental duplication or an ectopic ureter that is not obstructed (Figure 7). In girls, this should prompt questions about continuous incontinence to evaluate for ectopic ureters distal to the urethral sphincter (e.g., “has she ever had a period of dryness?”). Lower moiety hydronephrosis may be caused by vesicoureteral reflux, UPJ obstruction, or ureteral obstruction secondary to an upper pole ureterocele. Sonographically, a ureterocele appears as a thin-walled cystic intravesical structure (Figure 2), which may contain debris suggestive of urine stasis or infection.
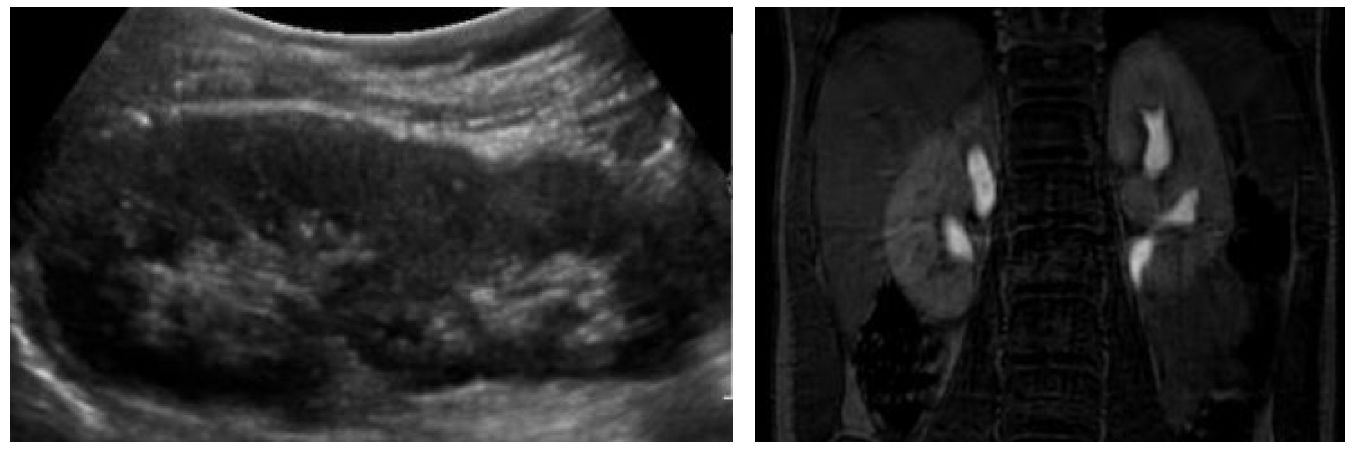
Figure 7 Continuous incontinence in a female due to bilateral ectopic ureters. A: Renal ultrasound demonstrates two healthy renal moieties without hydronephrosis. B: Magnetic resonance urography delineates the ureteral anatomy. In this case, both upper pole ureters entered the vagina.
Voiding Cystourethrography
VCUG is the study of choice to detect and characterize vesicoureteral reflux. In duplicated systems, reflux into the lower ureter only affects the inferolateral calyces, a radiographic pattern known as the “drooping lily sign” (Figure 8). VCUG may also demonstrate ureteroceles, which appear as filling defects in the bladder and are best seen during early filling (Figure 8) The dynamic nature of this modality yields information regarding bladder neck obstruction, ureterocele prolapse, and ureterocele eversion, which appears as a bladder diverticulum when the bladder is distended and suggests poor trigonal musculature. VCUG should be obtained if urinary tract dilation is seen on ultrasound to evaluate for high grade vesicoureteral reflux, and prior to urinary tract reconstruction as the presence of vesicoureteral reflux will influence surgical decision-making.
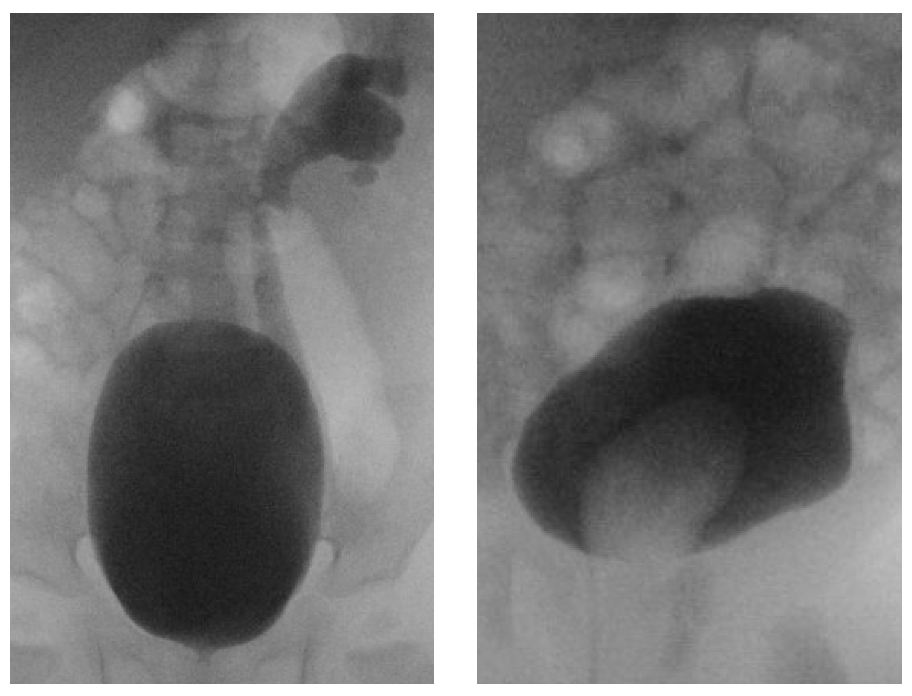
Figure 8 A: “Drooping lily sign.” Vesicoureteral reflux into the lower ureter fills the lower and interpolar calyces. The upper moiety collecting system does not fill. B: A ureterocele appears as a filling defect in the bladder.
Renal Scintigraphy
Nuclear renal scans demonstrate relative function and drainage of the renal moieties. In the non-pathologic duplex kidney, the relative function of the upper and lower moieties is one third and two thirds, respectively.51
Magnetic Resonance Urography
MRU provides precise anatomic detail in patients with complex anatomy that may be difficult to visualize with other modalities. MRU may be especially useful in tracing ectopic ureters (Figure 7). MRU is also useful in patients with ureteroceles that obscure other aspects of the bladder, or in patients with uterine, vaginal or other bladder anomalies.52 Computational software allows calculation of differential renal function and urine transit times, however this software is expensive, complex, and not yet widely available.53
Intravenous Urography
With other modalities available, IVU is now rarely performed. It can reveal duplication and the level of confluence, ureteral ectopia, and ureteroceles, among other anomalies. When upper moiety function is poor, associated pathology is not always demonstrated and delayed images may be helpful.
Migration and Fusion Anomalies
Abnormalities of ascent and fusion are commonly incidental findings. Non-dilated pelvic ectopic kidneys may be difficult to visualize on ultrasound, and absence of the kidney in the renal fossa may be misinterpreted as renal agenesis.24,54 In such cases the presence of ectopic functioning renal tissue is best demonstrated by dimercaptosuccinic acid (DMSA) renography.54 VCUG is not mandatory in the absence of urinary tract infections and hydronephrosis, as vesicoureteral reflux in this setting tends to be low grade and clinically insignificant.55 Symptoms such as renal colic and urinary tract infection generally denote additional pathology, such as high grade vesicoureteral reflux, nephrolithiasis, or UPJ obstruction, all of which are more common with renal ectopia and should prompt further evaluation (Figure 5)32,33,34
Treatment Options, Outcomes, and Complications
Duplication
Management of renal duplication depends upon the pathology at hand, and ranges from observation with or without antibiotic prophylaxis to minimally invasive surgery to open urinary tract reconstruction. In the absence of infection, obstruction, or urinary incontinence, duplication anomalies may be safely observed. Antibiotic prophylaxis is indicated in patients with duplication and ureteral dilation due to obstructing ectopic ureter, ureterocele, or dilating vesicoureteral reflux.56,57 Definitive urinary tract reconstruction can occur at the bladder level (lower tract) or at the kidney level (upper tract). A combined upper and lower tract approach is rarely necessary. The function of the renal moieties and the presence of vesicoureteral reflux are critical factors in formulating a surgical plan. If a patient has bladder level pathology including vesicoureteral reflux or a large or ectopic ureterocele, a lower tract approach is best.
Ureterocele Incision
Endoscopic decompression of the ureterocele was initially suggested in the early 1960s. The initial approach was to unroof the ureterocele, which was successful but incurred a high rate of de novo vesicoureteral reflux. In 1962, Zielinski suggested a low, medial, transverse incision at the base of the ureterocele.58 This provides adequate ureteral decompression while residual ureterocele tissue creates a functional flap valve to prevent de novo vesicoureteral reflux. The opening created should be intravesical to avoid obstruction by the tonically closed bladder neck. The incision can be completed with electrocautery, laser, or a cold knife. Successful initial decompression is seen in upwards of 90% of patients.59 More recently, a “watering can” approach has been advocated in the absence of infection wherein the ureterocele is punctured 10–20 times with a laser.60 This technique yields durable decompression with markedly reduced iatrogenic reflux, however the need for secondary procedures is less well-studied. For intravesical ureteroceles, regardless of technique, primary endoscopic decompression is frequently the only intervention needed.61,62,63 For ectopic ureteroceles, long-term outcomes are not as good; ureterocele decompression may be considered a temporizing operation as 50% to 80% will require secondary reconstruction due to symptomatic reflux or urinary incontinence.59,62,63
Subureteric Injection
As in single systems, vesicoureteral reflux in duplex systems can be managed with subureteric injection of bulking agents. Success was initially reported to be lower than in single systems, though subsequent analyses report comparable rates of success.64 The manufacturer of dextranomer-hyaluronic acid (Deflux®) has recently removed duplication as a contraindication. Resolution of vesicoureteral reflux after subureteric injection is related to age, sex, degree and timing of reflux, and is discussed in detail in the following chapter dedicated to vesicoureteral reflux.
Ureteral Clipping
Ureteral clipping is a newly described technique for management of the obstructed, nonfunctioning upper moiety.65,66 The upper moiety ureter is laparoscopically transected, drained, and ligated near the kidney with the expectation that the nonfunctioning renal tissue atrophies. This technique avoids the risk of de novo reflux associated with ureterocele puncture and is less invasive than traditional reconstruction for an ectopic ureter but requires ongoing surveillance and carries risks include symptomatic obstruction, infection, and even pyonephrosis, and has not been widely adopted.
Lower Tract Reconstruction
The lower urinary tract may be approached intra- or extra-vesically. Extravesical operations such as ureteroureterostomy may be performed through an inguinal or Gibson incision, depending on patient age. The floor of the inguinal canal is opened, the perivesical space is developed and the ureters are found posterolateral to the medial umbilical ligament. Some will pre-place ureteral stents in nondilated ureters to aid in their identification. In males, care is taken to avoid damaging the vas deferens, which drapes over the ureters. Intravesical operations such as cross-trigonal ureteral reimplantation are performed through a Pfannenstiel incision. Laparoscopic approaches involve traditional port placement for pelvic surgery, including an umbilical camera port and left and right infraumbilical working ports.
Ureteroureterostomy
Ureteroureterostomy is an excellent solution when there is isolated upper or lower pole pathology, but not both.67 If there is isolated lower vesicoureteral reflux, the lower ureter can be anastomosed to the upper ureter in an end-to-side fashion. More commonly, an upper-to-lower ureteroureterostomy is performed in the setting of an ectopic ureter causing urinary incontinence or obstruction. Upper pole ureters that are obstructed by small intravesical ureteroceles may also be managed with ureteroureterostomy; large ureteroceles or ectopic ureteroceles may require an intravesical operation for formal ureterocele excision and bladder floor reconstruction.68 Ureteroureterostomy has several advantages. It avoids the complex dissection around the renal hilum required with heminephrectomy, and avoids the need to open the bladder or taper dilated ureters as is required with traditional ureteral reimplantation. If there is no ureteral dilation, the ureter should be spatulated to allow a widely patent anastomosis. Long-term success rates exceed 90% in experienced hands.69,70,71,72 Risks include ureteroureteral (“yo-yo”) reflux, damage to the recipient ureter, anastomotic stenosis, urine leak, and infection of the ureteral stump, all of which are rare. A proximal anastomosis with excision of the distal ureteral stump may decrease infection of the ureteral stump, and is easily performed laparoscopically.73,74 If the ureters share a common sheath, excision of the distal ureteral stump should not be aggressively pursued in order to avoid damaging the shared blood supply. Stents, if placed, may either traverse the anastomosis or remain in the recipient ureter.
Ureteral Reimplantation
Isolated vesicoureteral reflux in duplex systems is treated as it is in single systems. When vesicoureteral reflux is present in addition to an ectopic or obstructed ureter requiring intervention, ureteral reimplantation is traditionally the procedure of choice. Distally, duplicated ureters are often located within a common sheath and share a distal blood supply. In this case, ureters must be mobilized and reimplanted en bloc.75 An ectopic ureter will not necessarily share a blood supply with the lower pole ureter. In this case, the ureters may be reimplanted independently.76 Alternatively, a combination upper-to-lower ureteroureterostomy and lower ureteral reimplant may be performed.77,78
Ureters may be reimplanted via an intravesical or extravesical approach, with or without ureteral tailoring. Bilateral extravesical reimplantation is avoided due to the risk of pelvic plexus damage and urinary retention. Reimplantation of obstructed or ectopic ureters, ureteral tailoring, and excision of associated ureteroceles requires dismembering the ureter and opening the bladder mucosa, and many surgeons prefer an intravesical approach in this setting. Vesicoscopic approaches to ureteral reimplantation have been described, but long-term outcomes are yet to be reported and the traditional laparoscopic approach is extravesical.79 The success rate of common sheath reimplantation is over 95%, similar to single-system reimplantation.80,81
Excision of Ureterocele and Bladder Reconstruction
Ureteroceles not decompressed endoscopically, or large ureteroceles with underlying abnormal musculature, are sometimes addressed with open surgery. This may include excision or marsupialization of the ureterocele, bladder floor reconstruction, and/or bladder neck reconstruction.82 Excision of the ureterocele is often performed at the time of ureteral reimplantation. Redundant mucosa is excised taking care not to leave a mucosal flap which can obstruct the bladder. The detrusor defect may be is reconstructed to ensure adequate backing for the reimplanted ureter. Contralateral reflux can result, presumably due to trigonal distortion, though the clinical significance is unclear. When the ureterocele extends past the bladder neck there is a significant risk of stress urinary incontinence and bladder dysfunction. Prudent dissection is required, however even with meticulous technique voiding and continence outcomes are poor and are felt to be intrinsic rather than acquired.68,83
Cutaneous Ureterostomy
This is a temporizing operation performed in the setting of urinary tract infection in the young patient.16 It is indicated for the infected, obstructed upper pole ureter or the refluxing lower pole ureter with breakthrough urinary tract infections on antibiotic prophylaxis. The open surgical approach is similar to a ureteroureterostomy. The pathologic ureter(s) are incised or transected and marsupialized to the inguinal skin incision. In infants, the combination of small bladders and dilated ureters precludes an adequate ureteral tunnel, and reimplantation is usually delayed. Ureteral dilation often improves with diversion, which may obviate the need for tailoring at the time of reimplantation. Definitive reconstruction is performed when the patient is 1-2 years of age. Notably, diverting ureterostomy is an excellent option for managing ureteroceles when endoscopic equipment is not available.
Upper Tract Approach
Upper tract reconstruction is appropriate when there is no lower urinary tract pathology to be addressed. Even so, a lower tract approach is often considered due it its relative ease and success.84,85
Heminephrectomy
The indication for upper heminephrectomy a poorly functioning upper pole with associated urinary incontinence or upper pole pathology (e.g. pyelonephritis, stones). The upper pole ureter is divided and used for traction. The upper pole hilar vessels are ligated, and the nonviable renal parenchyma demarcates, and is excised. Alternatively, the upper pole parenchyma, which is often poorly vascularized and easily delineated from the healthy lower pole, can be excised and traced to the upper pole hilar vessels and ureter, which are then divided. This simplifies the hilar dissection but risks bleeding. The cut edge of renal parenchyma is covered with Gerota’s fascia and perirenal fat. Redundant upper pole ureter is removed but the residual ureteric stump is left in situ. Utmost care is taken not to damage blood supply to the lower pole parenchyma or ureter.
Loss of lower pole function is the most significant complication related to upper heminephrectomy, with reported rates as high as nine percent.84,86 This can be due to hilar injury or post-operative torsion of the remaining kidney, and is more common in children younger than 1 year. For fully mobilized kidneys, consider nephropexy to prevent torsion of the renal remnant. Additional complications include postoperative bleeding (<5%), urinoma (5–20%, more common in laparoscopic procedures, rarely clinically significant).87,88 While upper heminephrectomy was once the procedure of choice for the nonfunctional upper pole without lower pole reflux, practice patterns are shifting towards ureteroureterostomy which is a simpler operation with similar success rates and less risk to lower pole function.84,85
Lower heminephrectomy is less common. This is done for clinically significant pathology (e.g., infection, symptomatic obstruction, stones) in the setting of a nonfunctional lower moiety with preservation of the upper pole. This occurs secondary to UPJ obstruction or high-grade vesicoureteral reflux. This operation can be more challenging than upper heminephrectomy, as the lower pole nests within the upper pole making it difficult to separate. Complications, and outcomes are similar to upper pole heminephrectomy.89
Pyeloplasty, Pyelo-Ureterostomy, Proximal Ureteroureterostomy
As noted previously, ureteroureterostomy with a proximal anastomosis carries some advantages over a distal anastomosis. With a laparoscopic approach, a flank incision is avoided and there is negligible additional morbidity compared to a distal anastomosis.73,74 Lower pole UPJ obstruction necessitates an upper tract approach. The particular technique depends on the configuration of the collecting system. With incomplete duplication the lower ureter may be short, precluding a traditional pyeloplasty. A pyelo-ureterostomy is performed by creating a side-to-side or end-to-side anastomosis between the obstructed lower pole renal pelvis and the upper pole ureter.9,90 If there is concomitant lower pole reflux, an end-to-side anastomosis with drainage and ligation of the ureteral stump is best. In cases of complete duplication, traditional pyeloplasty is an alternative which avoids risks to the upper drainage system.9 Complications and outcomes are similar to ureteroureterostomy and traditional pyeloplasty.
Migration and Fusion Anomalies
Investigation of an uncomplicated ectopic or horseshoe kidney can reasonably be limited to ultrasound. If there is no hydronephrosis and the child is asymptomatic, additional workup is unnecessary. In the setting of an empty renal fossa, a DMSA scintigram may locate ectopic renal tissue.50 Additional investigations are indicated if there is hydronephrosis or a history of documented infection raising concern for vesicoureteral reflux. It is important to stress that the majority of patients are untroubled by their abnormally placed kidney, and surgical intervention should be confined to correcting coexisting pathology, obstruction, or reflux. When surgery is warranted, surgeons must anticipate the anomalous renal vasculature.
Key Points
- Duplication and migration anomalies are common, and most are clinically insignificant.
- Clinically significant pathology varies widely. Management focuses on the diagnosis, prevention, and treatment of urinary tract obstruction, infection, renal dysfunction, and voiding problems.
- In complete duplication, the upper ureter is prone to obstruction due to a ureterocele or ectopic insertion, whereas the lower ureter is prone to vesicoureteral reflux.
- Management of extravesical ureteroceles is complex, and there may be longstanding problems with voiding and continence.
- Consider upper and lower moiety renal function and the presence of vesicoureteral reflux when formulating a surgical plan in duplex systems.
- When surgery is warranted in renal ectopia, bear in mind that the renal vasculature will be aberrant.
Suggested Readings
- Ross JH, Kay R. Ureteropelvic junction obstruction in anomalous kidneys. Urol Clin North Am 1998; 25 (2): 219–225. DOI: 10.1016/S0094-0143(05)70010-0.
- Le H-K, Chiang G. Long-term Management of Ureterocele in Duplex Collecting Systems: Reconstruction Implications. Curr Urol Rep 2018; 19 (2). DOI: 10.1007/s11934-018-0758-3.
- Stanasel I, Peters CA. Ectopic Ureter, Ureterocele, and Ureteral Anomalies. 12th ed., Philadelphia, PA: Elsevier Saunders; 2020.
- VanderBrink BA, Reddy PP. Anomalies of the Upper Urinary Tract. 12th ed., Philadelphia, PA: Elsevier Saunders; 2020, DOI: 10.1016/b978-1-4160-6911-9.00117-1.
References
- Moore KL, Persaud MD, Torchia MG. Urogenital System. The Developing Human: Clinically Oriented Embryology. 11th ed. Philadelphia, PA: Elsevier; 2020. DOI: 10.5005/jp/books/10691_7.
- Tanagho EA. Embryologic basis for lower ureteral anomalies: A hypothesis. Urology 1976; 7 (5): 451–464. DOI: 10.1016/0090-4295(76)90179-5.
- Decter RM. Renal duplication and fusion anomalies. Pediatr Clin North Am 1997; 44 (5): 1323–1341. DOI: 10.1016/S0031-3955(05)70559-9.
- Williams H. Renal revision: from lobulation to duplication—what is normal? Arch Dis Child. Educ Pract 2007; 92 (5). DOI: 10.1136/adc.2007.126680.
- Kaplan WE, Nasrallah P, King LR. Reflux in complete duplication in children. J Urol 1978; 120 (2): 220–222. DOI: 10.1016/s0022-5347(17)57116-5.
- Whitaker J, Danks DM. A Study of the Inheritance of Duplication of the Kidneys and Ureters. J Urol 1966; 95 (2): 176–178. DOI: 10.1016/s0022-5347(17)63429-3.
- Atwell JD, Cook PL, Howell CJ. Familial incidence of bifid and double ureters. Arch Dis Child 1974; 49 (5): 390–393. DOI: 10.1136/adc.49.5.390.
- Joseph DB, Bauer SB, Colodny AH. Lower pole ureteropelvic junction obstruction and incomplete renal duplication. J Urol 1989; 141 (4): 896–899. DOI: 10.1016/s0022-5347(17)41044-5.
- Ross JH, Kay R. Ureteropelvic junction obstruction in anomalous kidneys. Urol Clin North Am 1998; 25 (2): 219–225. DOI: 10.1016/S0094-0143(05)70010-0.
- Weigert C. Ueber einige Bildungsfehler der Ureteren. Arch Für Pathol Anat Physiol Für Klin Med. 1877; 70 (4): 490–501. DOI: 10.1007/BF01935232.
- Meyer R. Zur Anatomie und Entwicklungsgeschichte der Ureterverdoppelung. Virchows Arch Path Anat 1907; 187: 408–434. DOI: 10.1007/BF01946114.
- Stanasel I, Peters CA. Ectopic Ureter, Ureterocele, and Ureteral Anomalies. 12th ed., Philadelphia, PA: Elsevier Saunders; 2020.
- Duicu C, Kiss E, Simu I. A Rare Case of Double-System With Ectopic Ureteral Openings Into Vagina. Front Pediatr 2018; 6 (176). DOI: 10.3389/fped.2018.00176.
- Gillenwater JY, Grayhack JT, Howards SS, editors. Adult and Pediatric Urology. 4th ed., Lippincott Williams & Wilkins; 2002, DOI: 10.1001/jama.1991.03460240115044.
- Plaire JC, Pope JCI, Kropp BP. Management of Ectopic Ureters: Experience With the Upper Tract Approach. J Urol 1997; 158 (3): 1245–1247. DOI: 10.1016/s0022-5347(01)64442-2.
- Coplen DE, Duckett JW. The Modern Approach to Ureteroceles. J Urol 1995; 153 (1): 166–171. DOI: 10.1097/00005392-199501000-00068.
- Chwalle R. The process of formation of cystic dilatation of the vesical end of the ureter and of diverticula at the ureteral ostium. Urol Cutan Rev 1927; 31: 499–504.
- Glassberg KI, Braren V, Duckett JW. Suggested Terminology for Duplex Systems, Ectopic Ureters and Ureteroceles. J Urol 1984; 132 (6): 1153–1154. DOI: 10.1016/S0022-5347(17)50072-5.
- Bolduc S, Upadhyay J, Restrepo R. The predictive value of diagnostic imaging for histological lesions of the upper poles in duplex systems with ureteroceles. BJU Int 2003; 91 (7): 678–682. DOI: 10.1046/j.1464-410x.2003.04247.x.
- Abrahamsson K, Hansson E, Hermansson G. Bladder dysfunction: an integral part of the ectopic ureterocele complex. J Urol 1998; 160 (4): 1468–1470. DOI: 10.1016/s0022-5347(01)62593-x.
- Fehrenbaker LG, Kelalis PP, Stickler GB. Vesicoureteral Reflux and Ureteral Duplication in Children. J Urol 1972; 107 (5): 862–864. DOI: 10.1016/S0022-5347(17)61160-1.
- Campbell MF. Renal Ectopy. J Urol 1930; 24 (2): 187–198. DOI: 10.1016/s0022-5347(17)72892-3.
- Yuksel A, Batukan C. Sonographic Findings of Fetuses with an Empty Renal Fossa and Normal Amniotic Fluid Volume. Fetal Diagn Ther 2004; 19 (6): 525–532. DOI: 10.1159/000080166.
- Sheih CP, Liu MB, Hung CS. Renal abnormalities in schoolchildren. Pediatrics 1989; 84 (6): 1086–1090. DOI: 10.1542/peds.84.6.1086.
- Mouriquand P, Panait N. Renal Fusions and Ectopia. Pediatric Surgery 2012 (12). DOI: 10.1016/b978-0-323-02842-4.50113-3.
- N’Guessen G, Douglas Stephens F. Congenital superior ectopic (thoracic) kidney. Urology 1984; 24 (3): 219–228. DOI: 10.1016/0090-4295(84)90346-7.
- Natsis K, Piagkou M, Skotsimara A. Horseshoe kidney: a review of anatomy and pathology. Surg Radiol Anat 2014; 36 (6): 517–526. DOI: 10.1007/s00276-013-1229-7.
- Kirkpatrick JJ, Leslie SW. Horseshoe Kidney. 2021. DOI: 10.53347/rid-18142.
- Dajani AM. Horseshoe kidney: a review of twenty-nine cases. Br J Urol 1966; 38 (4): 388–402. DOI: 10.1111/j.1464-410x.1966.tb09725.x.
- Khougali HS, Alawad OAMA, Farkas N. Bilateral pelvic kidneys with upper pole fusion and malrotation: a case report and review of the literature. J Med Case Reports 2021; 15 (1). DOI: 10.1186/s13256-021-02761-1.
- Guarino N, Tadini B, Camardi P. The incidence of associated urological abnormalities in children with renal ectopia. J Urol 2004; 172 (4, Supplement): 1757–1759. DOI: 10.1097/01.ju.0000138376.93343.74.
- Kramer SA, Kelalis PP. Ureteropelvic junction obstruction in children with renal ectopy. J Urol (Paris 1984; 90 (5): 331–336. DOI: 10.1016/s0094-0143(05)70009-4.
- Gleason PE, Kelalis PP, Husmann DA. Hydronephrosis in Renal Ectopia: Incidence, Etiology and Significance. J Urol 1994; 151 (6): 1660–1661. DOI: 10.1016/S0022-5347(17)35338-7.
- McDonald JH, McClellan DS. Crossed renal ectopia. Am J Surg 1957; 93 (6): 995–1002. DOI: 10.53347/rid-26773.
- Marshall FF, Freedman MT. Crossed Renal Ectopia. J Urol 1978; 119 (2): 188–191. DOI: 10.53347/rid-26773.
- Abeshouse BS, Bhisitkul I. Crossed Renal Ectopia With and Without Fusion. Urol Int 1959; 9 (2): 63–91. DOI: 10.1159/000277442.
- VanderBrink BA, Reddy PP. Anomalies of the Upper Urinary Tract. 12th ed., Philadelphia, PA: Elsevier Saunders; 2020, DOI: 10.1016/b978-1-4160-6911-9.00117-1.
- CMA B, JAE W, GMA B. Urological and Nephrological Findings of Renal Ectopia. J Urol 2010; 183 (4): 1574–1578. DOI: 10.1016/j.juro.2009.12.041.
- Glodny B, Petersen J, Hofmann KJ. Kidney fusion anomalies revisited: clinical and radiological analysis of 209 cases of crossed fused ectopia and horseshoe kidney. BJU Int 2009; 103 (2): 224–235. DOI: 10.1111/j.1464-410X.2008.07912.x.
- Cunningham BK, Khromykh A, Martinez AF. Analysis of Renal Anomalies in VACTERL Association. Birt Defects Res A Clin Mol Teratol 2014; 100 (10): 801–805. DOI: 10.1002/bdra.23302.
- Je B-K, Kim HK, Horn PS. Incidence and Spectrum of Renal Complications and Extrarenal Diseases and Syndromes in 380 Children and Young Adults With Horseshoe Kidney. Am J Roentgenol 2015; 205 (6): 1306–1314. DOI: 10.2214/AJR.15.14625.
- Creatsas G, Malhotra N, Malhotra J. Vaginal agenesis associated with renal ectopia. Adolesc Pediatr Gynecol 1990; 3 (2): 103–105. DOI: 10.1016/S0932-8610(12)80191-5.
- Afshar K, Papanikolaou F, Malek R. Vesicoureteral reflux and complete ureteral duplication. conservative or surgical management? J Urol 2005; 173 (5): 1725–1727. DOI: 10.1097/01.ju.0000154164.99648.ee.
- Bisset G, Strife J. The duplex collecting system in girls with urinary tract infection: prevalence and significance. Am J Roentgenol 1987; 148 (3): 497–500. DOI: 10.2214/ajr.148.3.497.
- Stokland E, Jodal U, Sixt R. Uncomplicated duplex kidney and DMSA scintigraphy in children with urinary tract infection. Pediatr Radiol 2007; 37 (8): 826–828. DOI: 10.1007/s00247-007-0518-x.
- Hanson GR, Gatti JM, Gittes GK. Diagnosis of ectopic ureter as a cause of urinary incontinence. J Pediatr Urol 2007; 3 (1): 53–57. DOI: 10.1016/j.jpurol.2005.06.009.
- Gao Z, Wu J, Lin C, Men C. Transperitoneal Laparoscopic Heminephrectomy in Duplex Kidney: Our Initial Experience. Urology 2011; 77 (1): 231–236. DOI: 10.1016/j.urology.2010.02.002.
- Connolly JO, Chan MMY, Neild GH. Congenital Anomalies of the Kidney and Urinary Tract. Comprehensive Clinical Nephrology. 6th ed. Elsevier;2019:50,607-625.e1; . DOI: 10.1007/978-3-319-29219-9.
- Vergani P, Ceruti P, Locatelli A. Accuracy of prenatal ultrasonographic diagnosis of duplex renal system. J Ultrasound Med 1999; 18 (7): 463–467. DOI: 10.7863/jum.1999.18.7.463.
- Privett JTJ, Jeans WD, Roylance J. The incidence and importance of renal duplication. Clin Radiol 1976; 27 (4): 521–530. DOI: 10.1016/s0009-9260(76)80121-3.
- Dickerson EC, Dillman S JR, E.A.. Pediatric MR Urography: Indications, Techniques, and Approach to Review. Radiogr Rev Publ Radiol Soc N Am Inc 2015; 35 (4): 1208–1230. DOI: 10.1148/rg.2015140223.
- Jones RA, Perez-Brayfield MR, Kirsch AJ. Renal transit time with MR urography in children. Radiology 2004; 233 (1): 41–50. DOI: 10.1148/radiol.2331031117.
- Chow JS, Benson CB, Lebowitz RL. The Clinical Significance of an Empty Renal Fossa on Prenatal Sonography. J Ultrasound Med 2005; 24 (8): 1049–1054. DOI: 10.7863/jum.2005.24.8.1049.
- Elmacı AM, Dönmez Mİ, Soran M. Is voiding cystourethrography necessary for evaluating unilateral ectopic pelvic kidney? Turk J Urol 2019; 45 (Suppl 1). DOI: 10.5152/tud.2019.72798.
- Visuri S, Jahnukainen T, Taskinen S. Prenatal complicated duplex collecting system and ureterocele - important risk factors for urinary tract infection. J Pediatr Surg 2018; 53 (4): 813–817. DOI: 10.1016/j.jpedsurg.2017.05.007.
- Bascietto F, Khalil A, Rizzo G. Prenatal imaging features and postnatal outcomes of isolated fetal duplex renal collecting system: A systematic review and meta-analysis. Prenat Diagn 2020; 40 (4): 424–431. DOI: 10.1002/pd.5622.
- Zielínski J. Avoidance of Vesicoureteral Reflux after Transurethral Ureteral Meatotomy for Ureterocele. J Urol 1962; 88 (3): 386–386. DOI: 10.1016/S0022-5347(17)64805-5.
- Meir DB, CJTDAE S, Rao P. Does the endoscopic technique of ureterocele incision matter? J Urol 2004; 172 (2): 684–686. DOI: 10.1097/01.ju.0000129228.92805.31.
- Palmer BW, Greger H, Mannas DB. Comparison of Endoscopic Ureterocele Decompression Techniques. Preliminary Experience—Is the Watering Can Puncture Superior? J Urol 2011; 186 (4S): 1700–1704. DOI: 10.1016/j.juro.2011.04.007.
- Jelloul L, Berger D, Frey P. Endoscopic management of ureteroceles in children. Eur Urol 1997; 32 (3): 321–326. DOI: 10.1159/000019704.
- Cooper CS, Passerini-Glazel G, Hutcheson JC. Long-term followup of endoscopic incision of ureteroceles: intravesical versus extravesical. J Urol 2000; 164(3. DOI: 10.1097/00005392-200009020-00045.
- Renzo DD, Ellsworth PI, Caldamone AA. Transurethral Puncture for Ureterocele—Which Factors Dictate Outcomes? J Urol 1620; 2010;184(4. DOI: 10.1016/j.juro.2010.04.023.
- Hensle TW, Reiley EA, Ritch C. The clinical utility and safety of the endoscopic treatment of vesicoureteral reflux in patients with duplex ureters. J Pediatr Urol 2010; 6 (1): 15–22. DOI: 10.1016/j.jpurol.2009.05.015.
- Lopes RI, Fernandez N, Koyle MA. Clinical Outcomes of the Upper Urinary Tract after Ureteral Clipping for Treatment of Low Functioning or Nonfunctioning Renal Moieties. J Urol 2018; 199 (2): 558–564. DOI: 10.1016/j.juro.2017.09.080.
- Lopes RI, Mello MF, Koyle MA. Ureteral clipping for the treatment of a non-functioning upper kidney moiety associated with a massive ureterocele: step-by-step description of a novel technique. J Pediatr Urol 2019; 15 (3): 284–285. DOI: 10.1016/j.jpurol.2019.03.005.
- Buchtel HA. Uretero-ureterostomy. J Urol 1965; 93 (2): 153–157. DOI: 10.1016/s0022-5347(17)63740-6.
- Le H-K, Chiang G. Long-term Management of Ureterocele in Duplex Collecting Systems: Reconstruction Implications. Curr Urol Rep 2018; 19 (2). DOI: 10.1007/s11934-018-0758-3.
- Bracci U, Miano L, Lauretti C. Ureteroureterostomy in Complete Ureteral Duplication. Eur Urol 1979; 5: 347–351. DOI: 10.1159/000473152.
- Huisman TK, Kaplan GW, Brock WA. Ipsilateral Ureteroureterostomy and Pyeloureterostomy: A Review of 15 Years of Experience With 25 Patients. J Urol 1987; 138 (5): 1207–1210. DOI: 10.1016/S0022-5347(17)43551-8.
- Grimsby GM, Merchant Z, Jacobs MA. Laparoscopic-Assisted Ureteroureterostomy for Duplication Anomalies in Children. J Endourol 2014; 28 (10): 1173–1177. DOI: 10.1089/end.2014.0113.
- Abdelhalim A, Chamberlin JD, Truong H. Ipsilateral ureteroureterostomy for ureteral duplication anomalies: predictors of adverse outcomes. J Pediatr Urol 2019; 15 (5). DOI: 10.1016/j.jpurol.2019.05.016.
- Lee YS, Hah YS, Kim M-J. Factors Associated with Complications of the Ureteral Stump After Proximal Ureteroureterostomy. J Urol 2012; 188 (5): 1890–1894. DOI: 10.1016/j.juro.2012.07.015.
- Ellison JS, Lendvay TS. Robot-assisted ureteroureterostomy in pediatric patients: current perspectives. Robot Surg Res Rev 2017; 4: 45–55. DOI: 10.2147/RSRR.S99536.
- Belman AB, Filmer RB, King LR. Surgical Management of Duplication of the Collecting System. J Urol 1974; 112 (3): 316–321. DOI: 10.1016/S0022-5347(17)59718-9.
- Marshall S. Reimplantation of the Dilated Ectopic Ureter of the Duplex System as a Separate Unit. J Urol 1986; 135 (3): 574–575. DOI: 10.1016/S0022-5347(17)45743-0.
- Amar AD, Egan RM, Das S. Ipsilateral Ureteroureterostomy Combined with Ureteral Reimplantation for Treatment of Disease in both Ureters in a Child with Complete Ureteral Duplication. J Urol 1981; 125 (4): 581–582. DOI: 10.1016/s0022-5347(17)55111-3.
- Lashley DB, McAleer IM, Kaplan GW. Ipsilateral ureteroureterostomy for the treatment of vesicoureteral reflux or obstruction associated with complete ureteral duplication. J Urol 2001; 165 (2): 552–554. DOI: 10.1097/00005392-200102000-00067.
- Kanojia R, Pandey A, Bawa M. Vesicoscopic common sheath cohen’s reimplant in duplex ureters. J Urol 2021; 206 (Supplement 3, V03-03). DOI: 10.1097/JU.0000000000001991.03.
- Ellsworth PI, Lim DJ, Walker RD. Common sheath reimplantation yields excellent results in the treatment of vesicoureteral reflux in duplicated collecting systems. J Urol 1996; 155 (4): 1407–1409. DOI: 10.1097/00005392-199604000-00090.
- Barrieras D, Lapointe S, Houle A-M. Is Common Sheath Extravesical Reimplantation an Effective Technique to Correct Reflux in Duplicated Collecting Systems? J Urol 2003; 170 (4 Part 2): 1545–1547. DOI: 10.1097/01.ju.0000084149.02826.64.
- Lewis JM, Cheng EY, Campbell JB. Complete Excision or Marsupialization of Ureteroceles: Does Choice of Surgical Approach Affect Outcome? J Urol 2008; 180 (4S): 1819–1823. DOI: 10.1016/j.juro.2008.04.078.
- Holmes NM, Coplen DE, Strand W. Is Bladder Dysfunction and Incontinence Associated with Ureteroceles Congenital or Acquired? J Urol 2002; 168 (2): 718–719. DOI: 10.1016/S0022-5347(05)64732-5.
- Michaud JE, Akhavan A. Upper Pole Heminephrectomy Versus Lower Pole Ureteroureterostomy for Ectopic Upper Pole Ureters. Curr Urol Rep 2017; 18 (3). DOI: 10.1007/s11934-017-0664-0.
- Sheth KR, White JT, Janzen N. Evaluating Outcomes of Differential Surgical Management of Nonfunctioning Upper Pole Renal Moieties in Duplex Collecting Systems. Urology 2019; 123: 198–203. DOI: 10.1016/j.urology.2018.06.028.
- Gundeti MS, Ransley PG, Duffy PG. Renal outcome following heminephrectomy for duplex kidney. J Urol 2005; 173 (5): 1743–1744. DOI: 10.1097/01.ju.0000154163.67420.4d.
- Wallis MC, Khoury AE, Lorenzo AJ. Outcome Analysis of Retroperitoneal Laparoscopic Heminephrectomy in Children. J Urol 2006; 175 (6): 2277–2282. DOI: 10.1016/S0022-5347(06)00338-7.
- You D, Bang JK, Shim M. Analysis of the late outcome of laparoscopic heminephrectomy in children with duplex kidneys. BJU Int 2010; 106 (4): 250–254. DOI: 10.1111/j.1464-410x.2009.09038.x.
- Singh RR, Wagener S, Chandran H. Laparoscopic management and outcomes in non-functioning moieties of duplex kidneys in children. J Pediatr Urol 2010; 6 (1): 66–69. DOI: 10.1016/j.jpurol.2009.04.005.
- Leclair M-D, Vidal I, Suply E. Retroperitoneal Laparoscopic Heminephrectomy in Duplex Kidney in Infants and Children: A 15-Year Experience. Eur Urol 2009; 56 (2): 385–391. DOI: 10.1016/j.eururo.2008.07.015.
- Lowe GJ, Canon SJ, Jayanthi VR. Laparoscopic reconstructive options for obstruction in children with duplex renal anomalies. BJU Int 2008; 101 (2): 227–230. DOI: 10.1111/j.1464-410X.2007.07106.x.
Última actualización: 2024-03-17 22:39