51: Future Developments in Stone Management
This chapter will take approximately 18 minutes to read.
Introduction
The past two decades have seen a dramatic rise in pediatric kidney stone incidence, with adolescents representing the fasting growing age demographic across the lifespan of this disease.1 Because children and young adults face unique challenges in terms of care delivery, risks of treatment, and underlying disease processes, advances in kidney stone disease in terms of miniaturization of equipment, reducing ionizing radiation exposure, and improving care for patients with genetic predisposition for urinary stone disease may preferentially benefit pediatric patients. This chapter will review advances not only in surgical equipment but also diagnostics, therapeutics, and care delivery.
Diagnosis and Initial Evaluation
While ultrasound (US) remains the preferred first-line imaging strategy for most children with suspected nephrolithiasis, this imaging modality has limitations in terms of both operator dependence, availability, and accuracy.2,3 Meanwhile, computed tomography (CT) is the gold-standard imaging modality for diagnostic accuracy but carries high risks of ionizing radiation exposure, risks that are further compounded in the pediatric population.4 Innovations within the sphere of diagnostic imaging include enhancing US accuracy, reducing CT-based radiation exposure, and enhancing additional functionality such as stone propulsion.
US Accuracy
US relies upon sonographic properties at the stone-urine interface to produce the classic image of an echogenic focus on B-mode US. Additionally, deflection of the acoustic waves at the echogenic foci can produce a posterior acoustic shadow while application of Doppler settings can create a twinkle artifact over the stone.5 Both techniques can improve upon the accuracy of US imaging.6 Furthermore, the posterior acoustic shadow has the potential to provide a more accurate size estimate of the urinary calculus.7 However, at present imaging modalities to capture and enhance these findings are not well standardized nor is the identification and reporting of these measures standardized in any way and further evaluations to optimize present technologies and understanding of US findings are needed. Beyond optimization of current imaging technologies, modifications in the acoustic properties of the ultrasonic equipment may produce more distinct imaging characteristics of urinary stones. One such example, currently used on a research platform, is Stone-Mode (i.e., “S-mode™”) ultrasonic imaging. S-mode™ imaging relies on a high frequency transducer to optimize the visual interface between the stone and surrounding tissue and enhance the appearance of the acoustic shadow. Use of post-processing algorithms which tend to blur the images between the dense stone and posterior shadow are minimized, resulting in a sharper image of the calculus at a trade-off of diminished soft-tissue imaging.8
Low Dose CT Scans
Although enhancing US imaging is attractive to minimize ionizing radiation in children with nephrolithiasis, CT will likely remain a key imaging modality in the diagnoses of nephrolithiasis for years to come. Several facets of current stone-protocol CT algorithms, such as tube current, tube voltage, and gantry time, may be reduced in order to lower ionizing radiation dose during these studies.9 While low dose CT could hardly be classified as a “future technology,” given the vast amount of literature currently supporting its use, clearly the future of stone management could be improved by enhance the uptake of this technology.10 In this sense, the focus for low dose CT should be in strategies to improve provider education and decision-support incorporated into the electronic health record. Similar strategies have been utilized to produce sustainable radiation-stewardship practices within pediatric emergency departments.11
Imaging Propulsion
The use of ultrasonic propulsion in kidney stone disease was first reported in human trials in 2016.12,13 This technology, which harnesses focused acoustic energy via a transcutaneous probe, is able to propel kidney stone within the renal collecting system (Figure 1). Applications of this technology include repositioning obstructive kidney stones away from the ureteropelvic junction, repositioning stones into a more favorable treatment location (i.e., lower to upper pole), distinguishing between a small cluster of calculi and a dominant large calculus, and encouraging the passage of smaller fragments post-treatment. Human feasibility trials noted stone movement in 14 out of 15 individuals, including one who reported immediate relief upon repositioning of a partially obstructing calculus.12 Intra-operative use of this technology confirmed movement of calculi visually during ureteroscopy, confirming a proof-of-principle approach to repositioning calculi for easier endoscopic treatment.14 Although this technology has not, as the time of this writing, been applied in pediatric patients, the lack of ionizing radiation and opportunities to improve both diagnostic and therapeutic approaches make this an attractive future technology in the space of pediatric nephrolithiasis.
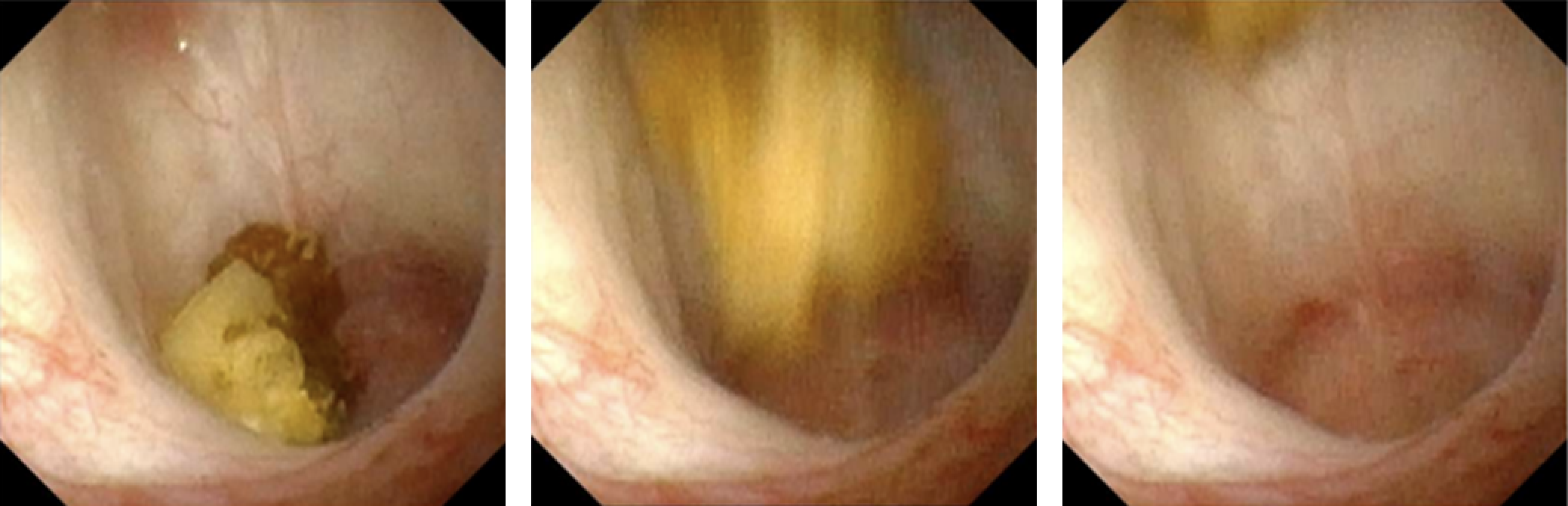
Figure 1 Real-time ureteroscopic image of in vivo propulsion of a 7 mm calyceal calculus via extracorporeal propulsion. Figure courtesy of Michael Bailey and Barbrina Dunmire at University of Washington.
Metabolic and Genetic Evaluation
While there has been little in the advancement of traditional evaluations for evaluations of kidney stone risk and underlying pathology (i.e., serum or urine studies), advances in genetic sequencing have expanded the opportunities for genetic testing in the realm of early-onset kidney stone disease. Genetic testing is not required for the diagnosis of certain monogenic kidney stone diseases with pathognomonic calculi (i.e., cystinuria or adenine phosphoribosyltrasnferase).15 However, genetic testing has been shown to detect potential monogenic sources of kidney stone disease in up to 20% of children referred to a tertiary kidney stone center.16 Practical issues surrounding the diagnostic strategy of genetic testing include the actionable information to be used based on the testing results and interpretation of variances of uncertain significance. In regards to the former issue, many genes on the most-reference multi-gene panels (often > 30) manifest in a multi-system manner, so that the diagnosis may be suspected, if not known, prior to genetic testing. Furthermore, in the majority of these monogenic kidney stone diseases, a focused treatment does not exist and is mostly driven by the clinical context. Nonetheless, early detection diseases such as primary hyperoxaluria may warrant testing in scenarios where the index of suspicion is high, such as younger patients or those with a strong family history of nephrolithiasis.17 Notably, approximately one-third of reported mutations are novel, raising both questions surrounding the pathological implications of these variances of uncertain significance as well as the potential to further expand upon the knowledge of genetically-based kidney stone disease. In such cases, bioinformatics analysis including software modeling to predict protein structure and potential pathology may inform interpretations of uncertain results.18 In summary, genetic evaluations may be useful especially when evaluating for potentially treatable sources of monogenic stone disease in high-risk populations, and collaboration with geneticists can prove invaluable when managing variances of uncertain significance or counseling.
Prevention
Reminder Technologies
Irrespective of novel therapeutic development for secondary kidney stone prevention, dietary and fluid intake measures remain the mainstays of preventative strategies.19 However, clinicians and patients alike recognize challenges to adherence to these recommendations, especially to high volumes of fluid intake daily. Several novel technologies, such as “smart” water bottles with the ability to send just-in-time electronic alerts to families, have been proposed as tools to improve fluid intake. Notably, only 20% adolescents provided with this technology still only met their fluid intake goals over the majority of a week-long study period.20 Other authors have trialed smart-phone applications and/or wearable technology to improve fluid intake experiences with mixed results.21,22 One promising avenue to enhance reminder technologies is the use of behavioral coaching, which is currently being explored in the Prevention of Urinary Stones with Hydration (PUSH) trial. As of this writing, the PUSH trial had finalized accrual but results were pending.23 Notably, there is an adolescent arm to the study, providing potential investigation in the pediatric experience, specifically. While the true value and impact of reminder technologies to improve adherence to secondary prevention of kidney stones remains elusive, one should consider that use of these technologies may still evolve in terms of format or interface while generational changes in technological uptake may further expand opportunities to leverage such innovations.
Medical Prevention
The most exciting advances in medical prevention for kidney stone disease include novel therapeutics in the form of targeted therapies as well as innovative avenues of pharmaceutical impact. The recent Food and Drug Association approval of lumasiran for Primary Hyperoxaluria Type 1 marks a landmark advancement as both the first targeted therapy for this disease as well as the first RNAi drug to be approved for use in nephro-urological disease.24 The medication targets messenger RNA encoding for glycolate oxidase, thereby inhibiting the conversion of glycolate to oxalate. It is administered subcutaneously every 1–3 months and has been approved for across the spectrum of the pediatric population, with weight-based dosing indicated for younger children, with those patients weighing less than 10 kilograms receiving monthly injections after a loading sequence and those heavier than 10 kilograms receiving injections every three months. Common side effects include injection site reactions (20%) and abdominal pain (15%), although few participants in the trials withdrew due to side effects.
Surgical Management
Amongst the multitude of recent advancements in the surgical management of urologic stone disease, several are particularly relevant for pediatric patients. Smaller body habitus, including the ureter itself, and susceptibility to radiation exposure are just some of the aspects of pediatric stone disease that differ from treatment in adult patients. New laser technologies have sought to decrease stone retropulsion and increase ablation rate, potentially leading to shorter operative times for pediatric ureteroscopy (URS). Miniaturized instrumentation for percutaneous nephrolithotomy (PCNL) may result in less morbidity and bleeding for pediatric patients. Furthermore, burst wave lithotripsy is a new technology that offers treatment for stone disease in the office setting, sparing children anesthesia and more difficult recovery.
Advances in Laser Technology
There have been several recent advances in laser technology including optimizations of the mainstay for laser lithotripsy, the holmium:YAG system, as well as a new thulium fiber laser (TFL) showing early promising results. Newer advances in holmium laser technology offer less movement of targeted stones during laser fragmentation and the ability to deliver more energy while the TFL offers similar benefits and in addition may allow for smaller laser fibers.
Holmium:YAG Modifications
The holmium:YAG system has been the gold standard for laser lithotripsy since its introduction, due to ease of use and favorable safety profile.25 Newer Moses and long pulse modifications of this technology alter this single, fixed pulse length approach. Long pulse modes deliver the same amount of energy over a longer time period, typically ranging from 500–1000 µs, reducing retropulsion at the expense of decreased energy delivery.26,27,28,29 The Moses effect describes a physical phenomenon that occurs when a holmium laser is fired in a fluid medium (Figure 2). This emitted energy is highly absorbed in water leading to the formation of a vapor tunnel. In a standard single pulse from a holmium laser, this energy transfer fails to reach the stone interface. Moses technology allows for energy to be delivered in two pulses; the first pulse delivers a small amount of energy causing a vapor channel to form while the second pulse delivers the majority of the energy that can now travel through the formed vapor channel to the target calculus.26 Moses technology has been shown to decrease operating time in the clinical setting due to decreased retropulsion and increased stone fragmentation efficiency.30,31
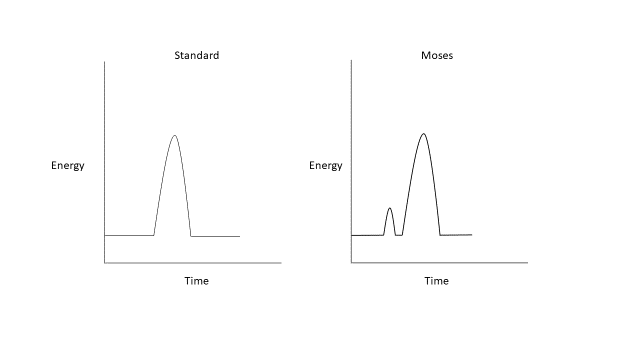
Figure 2 Standard and Moses holmium:YAG laser waveforms.
Thulium Fiber
TFL generates a laser beam more efficiently than holmium:YAG, relying on a diode laser that emits light within the absorption peak of thulium ions thereby exciting thulium ions within a thin silicon fiber with minimal loss of energy as heat, allowing for higher frequency ranges and broader ranges of pulse energy.25,32 Example settings used in the fragmentation of a renal stone as well as the TFL generator can be seen in Figure 3. Smaller laser fibers, as small as 150 µm and 50 µm in in vitro development, allow for improved irrigation flow and the potential to further miniaturize ureteroscopes.33 The TFL emits light at a wavelength of 1940 nm, even closer to water’s absorption peak of 1910 nm than the light emitted by holmium:YAG lasers. This leads to a favorable safety profile as the depth of penetration is reduced.34

Figure 3 Example settings of TFL, utilized for treatment of a renal calculus (example shown here during adult lithotripsy).
Multiple in vitro studies suggest that the TFL can produce faster ablation rates and potentially better stone clearance than the holmium:YAG system.35,36 However, there is some debate if these differences are clinically significant. Jaeger et al compared TFL to holmium:YAG during URS among 125 pediatric patients, 32 of which were treated with TFL. Patients treated with a TFL were less likely to have a retained stone fragment with no significant difference in operative time or complication rate, albeit with a longer lasering time in the TFL group.37 A meta-analysis of clinical studies in adults including nearly 1,700 patients showed several advantages with TFL as compared to holmium:YAG, including better operative times, laser utilization times, retropulsion, and stone clearance. There were no differences in ablation efficiency, total energy usage or hospital stay.38
Due to both the emitted energy being so similar to the absorption peak of water and the ability to operate at higher power and frequency settings heat and thermal injury are of greater concern with the TFL than with a holmium laser. Furthermore, simulated models have demonstrated that younger patients with smaller kidneys may be more susceptible to thermal injury during ureteroscopy for renal stones. However, these effects may be mitigated with the use of an access sheath and continuous pressurized irrigation39
Miniaturization of PCNL
Early experience with PCNL in pediatric patients was complicated by higher bleeding rates and a larger proportion of patients needing blood transfusions after their procedures due to the use of larger, adult sized instruments (up to 30 Fr).40,41 There have been significant advances in miniaturizing the instruments needed to perform PCNL; this has led to increased utilization of PCNL in the pediatric population with lower complication rates.42 Mini-PCNL was first described in 1998 by Jackman et al in an attempt to develop a less invasive PCNL technique that would decrease morbidity in young children.42 Notably, this innovation initially described in children has taken hold amongst the adult population as well.
Further efforts to miniaturize PCNL have resulted in ultra-mini PCNL (UMP) (11–13 Fr) and micro-PCNL (4.8 Fr). UMP can be accomplished with either a nephroscope or ureteroscope utilizing laser fragmentation and has shown equivalent stone clearance to mini-PCNL with longer operative times.43,44 Micro-PCNL represents the smallest access tract currently reported for miniaturized PCNL procedures, using an “all-seeing needle” which measures 4.85 Fr and has a lumen that can accommodate a 200 micron laser fiber.45 It is important to note that this technique does not allow for significant removal of stone fragments directly through the access tract.
There are multiple advantages to reducing the instrumentation size in PCNL including: decreased bleeding, decreased trauma to renal parenchyma and decreased pain related to the access tract. A recent systematic review sought to evaluate the efficacy and complications of minimally invasive PCNL techniques in pediatric patients.46 A total of 14 studies including 456 patients who underwent micro-PCNL or UMP were included. Mean stone size ranged from 12 to 16.5 mm. The stone free rate ranged from 80–100% and complications were seen in 14% of patients. 77% of complications were Clavien-Dindo grade I or II. Complications included hematuria, fever, urine leak, UTI, need for transfusion and 3 patients with renal pelvic perforation. Overall, the transfusion rate in this systematic review was 2.1%. Minimally invasive PCNL has proven to be safe for pediatric patients. However, there are some disadvantages including decreased visual clarity and inability to remove stones through the access tract for micro-PCNL. There has been some evidence of an increase in intra-renal pressure in mini-PCNL as well.47 With an increasing range in options for the surgical management of pediatric renal stone disease UMP and micro-PCNL represent an attractive option that should be considered in patients with a moderate renal stone burden.
Advances in Ultrasonic Stone Fragmentation: Burst Wave Lithotripsy
Although historically, SWL has been used as a first line therapy to fragment stones in the pediatric population the procedure requires general anesthesia and with more recent advances in endoscopic techniques has fallen out of favor in many situations: particularly in patients with larger stone burdens, harder stone compositions, increased skin to stone distance and stone disease in the lower pole of the kidney.48 SWL involves delivering single cycle of energy at a slow rate (0.5–3 Hz) to fragment stones. Burst wave lithotripsy (BWL) is a relatively new modality being explored that uses multicycle sinusoidal bursts of focused ultrasound pulses to fracture stones rather than the single compression/tension cycle created by SWL (Figure 4). BWL offers a potential therapy that could be used in an office setting or sedation room without the need for general anesthesia, which would be an attractive option for many pediatric patients.13
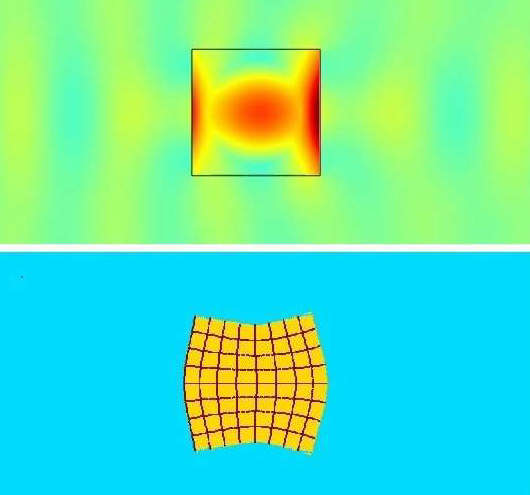
Figure 4 Simulation of burst wave lithotripsy (BWL) across a calculus interface. Top image shows the yellow and blue-green pressure waves moving across the stone, with 5-fold central stress amplification within the calculus due to the multiple pulse cycles of BWL. Bottom image demonstrates an exaggerated image of stress-induced stone distortion owing to the stress amplification previously described. Image courtesy of Oleg Sapozhnikov at University of Washington.
There are several important differences in the properties generated by BWL when compared with traditional SWL. For SWL an approximately 5 µs pulse repeats every 0.33 to 2 seconds resulting in 180 shockwaves per minute. BWL is delivered using 10–100 cycles at a time and has been investigated at a range of 300–500 kHz in humans.13 The peak amplitude of an SWL wave form is approximately 10-fold greater than BWL. However, BWL can deliver the same amount of energy due to using so many more cycles than the single SWL pulse of energy. Furthermore, while SWL creates a cloud cavitation in a focal area that can cause tissue injury, BWL there is milder growth of cavitation bubbles. Bubble clouds are more diffuse and do not typically undergo violent collapse, potentially minimizing cellular damage.49 Although there is some evidence that the more diffuse bubble clouds generated in BWL can lead to shielding of the targeted stone from subsequent pulses, BWL may overcome this limitation by scaling the delivery of energy.50 One model showed that using specific frequencies for different stone sizes can amplify the internal stress in a stone generated by BWL, an option not available in standard SWL.51
There is limited data on the use of BWL in the clinical setting, and it should be noted that this treatment has not yet been studied in children.52 Current clinical experiences are summarized in Table 1. The largest currently reported trial, of 19 patients, treated stones < 12 mm for up to 10 minutes with BWL. A median of 90% of stone volume was completely comminuted in 10 minutes and 39% of stone targets were fragmented with all pieces <2 mm within 10 minutes.53 Another study treated 13 awake and unanesthetized patients with both ultrasonic propulsion and BWL, with 70% reported stone clearance and a mean pain score of 1.2/10 during treatment.54
Table 1 Summary of PubMed search for burst wave lithotripsy evaluations in human trials.
| Year | Author | Title | Key Results | |
|---|---|---|---|---|
| 2021 | Harper | First In-Human Burst Wave Lithotripsy for Kidney Stone Comminution: Initial Two Case Studies54 | Patient A had successful BWL for renal stone observed via URS. Patient B had awake BWL for 7.5 mm UVJ stone, tolerated procedure well, stone passed POD 15. | |
| 2022 | Harper | Fragmentation of Stones by Burst Wave Lithotripsy in the First 19 Humans10 | 21 of 23 stones fragmented. Median stone comminution 90%. Complete fragmentation <10 minutes for 9/23 stones. | |
| 2022 | Hall | First Series Using Ultrasonic Propulsion and Burst Wave Lithotripsy to Treat Ureteral Stones11 | 13 patients treated with BWL in conjunction with ultrasonic propulsion. 70% of patients who underwent awake BWL passed stone. Mean pain score during BWL 1.2/10. |
Conclusions
In many ways—such as optimized imaging strategies, RNA-inhibitors for primary hyperoxaluria, and surgical advances in laser technology and miniaturized PCNL—the future of pediatric kidney stone management is already here. However, these technologies must be further understood in terms of most effective clinical use, questions which are ripe for comparative effectiveness research. Other technological advances, such as imaging propulsion and BWL, remain on the horizon of management yet offer promising roads to treatments in children within the next several decades.
References
- Tasian GE, Ross ME, Song L, Sas DJ, Keren R, Denburg MR, et al.. Annual Incidence of Nephrolithiasis among Children and Adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol 2016; 11 (3): 488–496. DOI: 10.2215/cjn.07610715.
- Roberson NP, Dillman JR, O’Hara SM, DeFoor WR, Reddy PP, Giordano RM, et al.. Comparison of ultrasound versus computed tomography for the detection of kidney stones in the pediatric population: a clinical effectiveness study. Pediatr Radiol 2018; 48 (7): 962–972. DOI: 10.1007/s00247-018-4099-7.
- Ellison JS, Thakrar P. The Role of Imaging in Management of Stone Disease. Diagnosis and Management of Pediatric. Nephrolithiasis: Springer; 2022. DOI: 10.1007/978-3-031-07594-0\\_8.
- Frush DP. Pediatric CT: practical approach to diminish the radiation dose. Pediatr Radiol 2002; 32 (10): 714–717. DOI: 10.1007/s00247-002-0797-1.
- Dai JC, Bailey MR, Sorensen MD, Harper JD. Innovations in Ultrasound Technology in the Management of Kidney Stones. Urol Clin North Am 2019; 46 (2): 273–285. DOI: 10.1016/j.ucl.2018.12.009.
- Masch WR, Cohan RH, Ellis JH, Dillman JR, Rubin JM, Davenport MS. Clinical Effectiveness of Prospectively Reported Sonographic Twinkling Artifact for the Diagnosis of Renal Calculus in Patients Without Known Urolithiasis. AJR Am J Roentgenol 2016; 206 (2): 326–331. DOI: 10.2214/ajr.15.14998.
- Verhagen MV, Watson TA, Hickson M, Smeulders N, Humphries PD. Acoustic shadowing in pediatric kidney stone ultrasound: a retrospective study with non-enhanced computed tomography as reference standard. Pediatr Radiol 2019; 49 (6): 777–783. DOI: 10.1007/s00247-019-04372-x.
- Simon JC, Dunmire B, Bailey MR, Sorensen MD. Developing Complete Ultrasonic Management of Kidney Stones for Spaceflight. J Space Saf Eng 2016; 3 (2): 50–57. DOI: 10.1016/s2468-8967(16)30018-0.
- Lira D, Padole A, Kalra MK, Singh S. Tube Potential and CT Radiation Dose Optimization. AJR Am J Roentgenol 2015; 204 (1): W4–w10. DOI: 10.2214/ajr.14.13281.
- Niemann T, Kollmann T, Bongartz G. Diagnostic Performance of Low-Dose CT for the Detection of Urolithiasis: A Meta-Analysis. AJR Am J Roentgenol 2008; 191 (2): 396–401. DOI: 10.2214/ajr.07.3414.
- Ellison JS, Crowell CS, Clifton H, Whitlock K, Haaland W, Chen T, et al.. A clinical pathway to minimize computed tomography for suspected nephrolithiasis in children. J Pediatr Urol 2019; 15 (5): 518.e1–518.e7. DOI: 10.1016/j.jpurol.2019.06.014.
- Harper JD, Cunitz BW, Dunmire B. Faculty Opinions recommendation of First in human clinical trial of ultrasonic propulsion of kidney stones. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2016; 195: 956, DOI: 10.3410/f.725901820.793511249.
- Raskolnikov D, Bailey MR, Harper JD. Recent Advances in the Science of Burst Wave Lithotripsy and Ultrasonic Propulsion. BME Front 2022; 2022. DOI: 10.34133/2022/9847952.
- Dai JC, Sorensen MD, Chang HC, Samson PC, Dunmire B, Cunitz BW, et al.. Quantitative Assessment of Effectiveness of Ultrasonic Propulsion of Kidney Stones. J Endourol 2019; 33 (10): 850–857. DOI: 10.1089/end.2019.0340.
- Goldstein R, Goldfarb DS. Early Recognition and Management of Rare Kidney Stone Disorders. Urol Nurs 2017; 37 (2): 81. DOI: 10.7257/1053-816x.2017.37.2.81.
- Daga A, Majmundar AJ, Braun DA, Gee HY, Lawson JA, Shril S, et al.. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int 2018; 93 (1): 204–213. DOI: 10.1016/j.kint.2017.06.025.
- Langman CB. A rational approach to the use of sophisticated genetic analyses of pediatric stone disease. Kidney Int 2018; 93 (1): 15–18. DOI: 10.1016/j.kint.2017.08.023.
- Ma Y, Lv H, Wang J, Tan J. Heterozygous mutation of SLC34A1 in patients with hypophosphatemic kidney stones and osteoporosis: a case report. J Int Med Res 2020; 48 (3): 030006051989614. DOI: 10.1177/0300060519896146.
- Tasian GE, Copelovitch L. Evaluation and Medical Management of Kidney Stones in Children. J Urol 2014; 192 (5): 1329–1336. DOI: 10.1016/j.juro.2014.04.108.
- Tasian GE, Ross M, Song L. Ecological Momentary Assessment of Factors Associated with Water Intake Among Adolescents with Kidney Stone Disease. 2018. DOI: 10.1016/j.juro.2018.07.064.
- Conroy DE, West AB, Brunke-Reese D, Thomaz E, Streeper NM. Just-in-time adaptive intervention to promote fluid consumption in patients with kidney stones. Health Psychol 2020; 39 (12): 1062–1069. DOI: 10.1037/hea0001032.
- Wright HC, Alshara L, DiGennaro H, Kassis YE, Li J, Monga M, et al.. The impact of smart technology on adherence rates and fluid management in the prevention of kidney stones. Urolithiasis 2022; 50 (1): 29–36. DOI: 10.1007/s00240-021-01270-6.
- Scales CD, Desai AC, Harper JD, Lai HH, Maalouf NM, Reese PP, et al.. Prevention of Urinary Stones With Hydration (PUSH): Design and Rationale of a Clinical Trial. Am J Kidney Dis 2020; 77 (6): 898–906.e1. DOI: 10.1053/j.ajkd.2020.09.016.
- Garrelfs SF, Frishberg Y, Hulton SA. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N Engl J Med 2021; 385 (20): e69. DOI: 10.1056/nejmc2107661.
- Traxer O, Keller EX. Thulium fiber laser: the new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J Urol 2020; 38 (8): 1883–1894. DOI: 10.1007/s00345-019-02654-5.
- Aldoukhi AH, Black KM, Ghani KR. Emerging Laser Techniques for the Management of Stones. Urol Clin North Am 2019; 46 (2): 193–205. DOI: 10.1016/j.ucl.2018.12.005.
- Kang HW, Lee H, Teichman JMH, Oh J, Kim J, Welch AJ. Dependence of calculus retropulsion on pulse duration during HO: YAG laser lithotripsy. Lasers Surg Med 2006; 38 (8): 762–772. DOI: 10.1002/lsm.20376.
- Bell JR, Penniston KL, Nakada SY. In Vitro Comparison of Holmium Lasers: Evidence for Shorter Fragmentation Time and Decreased Retropulsion Using a Modern Variable-pulse Laser. Urology 2017; 107: 37–42. DOI: 10.1016/j.urology.2017.06.018.
- Kronenberg P, Traxer O. Update on lasers in urology 2014: current assessment on holmium:yttrium–aluminum–garnet (Ho:YAG) laser lithotripter settings and laser fibers. World J Urol 2015; 33 (4): 463–469. DOI: 10.1007/s00345-014-1395-1.
- Wang M, Shao Q, Zhu X, Wang Z, Zheng A. Efficiency and Clinical Outcomes of Moses Technology with Flexible Ureteroscopic Laser Lithotripsy for Treatment of Renal Calculus. Urol Int 2021; 105 (7-8): 587–593. DOI: 10.1159/000512054.
- Ibrahim A, Fahmy N, Carrier S, Elhilali M, Andonian S. Double-blinded prospective randomized clinical trial comparing regular and moses modes of holmium laser lithotripsy: Preliminary results. European Urology Supplements 2020; 17 (2): e1390. DOI: 10.1016/s1569-9056(18)31815-3.
- Panthier F, Doizi S, Berthe L, Traxer O. In vitro comparison of ablation rates between superpulsed thulium fiber laser and ho:Yag laser for endocorporeal lithotripsy. Eur Urol Open Sci 2020; 19: e1884–e1885. DOI: 10.1016/s2666-1683(20)33870-2.
- Khusid JA, Khargi R, Seiden B, Sadiq AS, Atallah WM, Gupta M. Thulium fiber laser utilization in urological surgery: A narrative review. Investig Clin Urol 2021; 62 (2): 136. DOI: 10.4111/icu.20200467.
- Taratkin M, Azilgareeva C, Cacciamani GE, Enikeev D. Thulium fiber laser in urology: physics made simple. Curr Opin Urol 2022; 32 (2): 166–172. DOI: 10.1097/mou.0000000000000967.
- Andreeva V, Vinarov A, Yaroslavsky I, Kovalenko A, Vybornov A, Rapoport L, et al.. Preclinical comparison of superpulse thulium fiber laser and a holmium:YAG laser for lithotripsy. World J Urol 2020; 38 (2): 497–503. DOI: 10.1007/s00345-019-02785-9.
- Jiang P, Okhunov Z, Afyouni AS, Ali SN, Sharifi H, Bhatt R, et al.. Comparison of Superpulse Thulium Fiber Laser vs. Holmium Laser for Ablation of Renal Calculi in an In-Vivo Porcine Model. J Endourol 2022. DOI: 10.1089/end.2022.0445.
- Jaeger CD, Nelson CP, Cilento BG, Logvinenko T, Kurtz MP. Comparing Pediatric Ureteroscopy Outcomes with SuperPulsed Thulium Fiber Laser and Low-Power Holmium:YAG Laser. J Urol 2022; 208 (2): 426–433. DOI: 10.1097/ju.0000000000002666.
- Chua ME, Bobrowski A, Ahmad I. Thulium fibre laser vs holmium: yttrium-aluminium-garnet laser lithotripsy for urolithiasis: meta-analysis of clinical studies. 2022. DOI: 10.1111/bju.15921.
- Ellison JS, MacConaghy B, Hall TL, Roberts WW, Maxwell AD. A simulated model for fluid and tissue heating during pediatric laser lithotripsy. J Pediatr Urol 2020; 16 (5): 626.e1–626.e8. DOI: 10.1016/j.jpurol.2020.07.014.
- Quhal F, Al Faddagh A, Silay MS, Straub M, Seitz C. Paediatric stone management: innovations and standards. Curr Opin Urol 2022; 32 (4): 420–424. DOI: 10.1097/mou.0000000000001004.
- Zeren S, Satar N, Bayazit Y, Bayazit AK, Payasli K, Özkeçeli R. Percutaneous Nephrolithotomy in the Management of Pediatric Renal Calculi. J Endourol 2002; 16 (2): 75–78. DOI: 10.1089/089277902753619546.
- Jackman SV, Hedican SP, Peters CA, Docimo SG. Percutaneous nephrolithotomy in infants and preschool age children: experience with a new technique. Urology 1998; 52 (4): 697–701. DOI: 10.1016/s0090-4295(98)00315-x.
- Wright A, Rukin N, Smith D, Rosette J De la, Somani BK. ‘Mini, ultra, micro’ – nomenclature and cost of these new minimally invasive percutaneous nephrolithotomy (PCNL) techniques. Ther Adv Urol 2016; 8 (2): 142–146. DOI: 10.1177/1756287215617674.
- Mishra DK, Bhatt S, Palaniappan S, Reddy TVK, Rajenthiran V, Sreeranga YL, et al.. Mini versus ultra-mini percutaneous nephrolithotomy in a paediatric population. Asian J Urol 2022; 9 (1): 75–80. DOI: 10.1016/j.ajur.2021.06.002.
- Desai MR, Sharma R, Mishra S, Sabnis RB, Stief C, Bader M. Single-Step Percutaneous Nephrolithotomy (Microperc): The Initial Clinical Report. J Urol 2011; 186 (1): 140–145. DOI: 10.1016/j.juro.2011.03.029.
- Jones P, Bennett G, Aboumarzouk OM, Griffin S, Somani BK. Role of Minimally Invasive Percutaneous Nephrolithotomy Techniques–Micro and Ultra-Mini PCNL (<15F) in the Pediatric Population: A Systematic Review. J Endourol 2017; 31 (9): 816–824. DOI: 10.1089/end.2017.0136.
- Loftus CJ, Hinck B, Makovey I, Sivalingam S, Monga M. Mini Versus Standard Percutaneous Nephrolithotomy: The Impact of Sheath Size on Intrarenal Pelvic Pressure and Infectious Complications in a Porcine Model. J Endourol 2018; 32 (4): 350–353. DOI: 10.1089/end.2017.0602.
- Silay MS, Ellison JS, Tailly T, Caione P. Update on Urinary Stones in Children: Current and Future Concepts in Surgical Treatment and Shockwave Lithotripsy. Eur Urol Focus 2017; 3 (2-3): 164–171. DOI: 10.1016/j.euf.2017.07.005.
- Maeda K, Colonius T. Bubble cloud dynamics in an ultrasound field. J Fluid Mech 2019; 862: 1105–1134. DOI: 10.1017/jfm.2018.968.
- Maeda K, Maxwell AD, Colonius T. Investigation of the Energy Shielding of Kidney Stones by Cavitation Bubble Clouds during Burst Wave Lithotripsy. Proceedings of the 10th International Symposium on Cavitation (CAV2018) 2018; 144: 626–630. DOI: 10.1115/1.861851_ch119.
- Sapozhnikov OA, Maxwell AD, Bailey MR. Maximizing mechanical stress in small urinary stones during burst wave lithotripsy. J Acoust Soc Am 2021; 150 (6): 4203–4212. DOI: 10.1121/10.0008902.
- Harper JD, Metzler I, Hall MK. Faculty Opinions recommendation of First In-Human Burst Wave Lithotripsy for Kidney Stone Comminution: Initial Two Case Studies. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2020. DOI: 10.3410/f.738685997.793585856.
- Harper JD, Lingeman JE, Sweet RM. Re: Fragmentation of Stones by Burst Wave Lithotripsy in the First 19 Humans. Eur Urol 2022; 82 (5): 569. DOI: 10.1016/j.eururo.2022.07.012.
- Hall MK, Thiel J, Dunmire B. First Series Using Ultrasonic Propulsion and Burst Wave Lithotripsy to Treat Ureteral Stones. Letter. J Urol 2022; 209 (2): 325–326. DOI: 10.1097/ju.0000000000003060.
Last updated: 2024-02-16 20:59