21: Bladder Exstrophy and Epispadias Complex
This chapter will take approximately 37 minutes to read.
Introduction
Bladder exstrophy is a rare genitourinary malformation that, when simply defined, refers to the eversion of the bladder to the outside of the body (Figure 1). It is a rare genitourinary malformation for which the management still challenges the field of pediatric urology. More completely, the Exstrophy-Epispadias Complex comprises many defects that range from isolated male or female epispadias, classic bladder exstrophy, and even cloacal exstrophy.
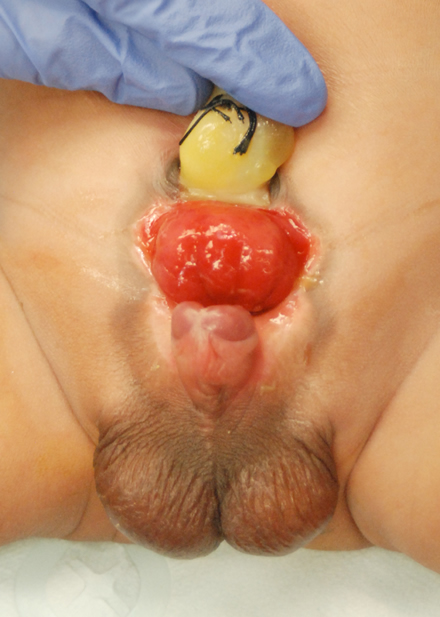
Figure 1 Newborn male with classic bladder exstrophy.
Incidence
The incidence of bladder exstrophy was originally estimated between 1:10,000 and 1:50,000 live births. However, more recent data from the International Clearinghouse for Birth Defects monitoring system and the Healthcare Cost and Utilization Project Nationwide Inpatient Sample from the United States estimated the incidence to be 2.15–3.3 per 100,000 live births. There is a male predominance with the male to female ratio has been reported between 2.3–6:1.
Embryology
Mesodermal ingrowth between the ectodermal and endodermal layers of the bilaminar cloacal membrane results in formation of the lower abdominal musculature and pelvic bones. After mesenchymal ingrowth occurs, downward growth of the rectal septum divides the cloaca into a bladder anteriorly and a rectum posteriorly. The genital tubercles migrate medially and fuse in the midline cephalad to the dorsal membrane before it perforates. The cloacal membrane is subject to premature rupture depending on the extent of the infraumbilical defect. The stage of development when the membrane rupture occurs determines whether bladder exstrophy, cloacal exstrophy, or epispadias results.
The most related theory of embryonic development in exstrophy, held by Marshall and Muecke, describes the basic defect as an abnormal lower overdevelopment of the cloacal membrane, which prevents the medial migration of the mesenchymal tissue. Therefore, proper development of the abdominal wall does not occur. The timing of the rupture of this cloacal defect determines the severity of the disorder. Central perforations resulting in classic exstrophy have the highest incidence (60%) whereas exstrophy variants account for 30% and cloacal exstrophy 10%.
Other theories are offered concerning the cause of the exstrophy-epispadias complex. Ambrose and O’Brian postulated that an abnormal development of the genital hillocks with fusion in the midline below rather than above the cloacal membrane result in the exstrophy defect.1 Another hypothesis describes an abnormal caudal insertion of the body stalk with failure of the interposition of the mesenchymal tissue in the midline. Because of this failure, translocation of the cloaca into the depths of the abdominal cavity does not occur. A cloacal membrane that remains in a superficial infraumbilical position represents an unstable embryonic state with a strong tendency to disintegrate. No one theory seems to elucidate all aspects of the complex seen clinically and further study is ongoing to fully describe the developmental process that ultimately forms the exstrophy-epispadias complex.
Inheritance
Evidence exists for a genetic predisposition for exstrophy and epispadias. The risk of recurrence of bladder exstrophy in a given family is approximately 1 in 100 , much greater than in the general population. There are many reports of twins with exstrophy. At the same time, however, there are also reports of identical twins with both having exstrophy and another set in which only one was affected. There are numerous cases of nonidentical twins in which only one sibling was affected. These twin sets were found in both male and female pairs.2,3 Concordance analyses of twins with bladder exstrophy-epispadias complex also suggest a genetic etiology. A report of 151 families with exstrophy-epispadias complex found 4 multiplex families for a rate of 2.7%. The likelihood of an exstrophic parent producing a child with exstrophy is about 1:70 live births or 500 times the risk for the general population.3
Many efforts have been made to understand the possible etiologies of the exstrophy-epispadias complex. The early developmental hormonal milieu associated with in vitro fertilization has been postulated to be involved based on studies that showed a 7.5-fold increase in exstrophy and cloacal exstrophy associated with the use of assisted reproductive technology such as introcystoplasmic sperm injection. Another epidemiology study showed an increased rate of exstrophy-epispadias complex births to women who underwent in vitro fertilization. A more recent study from a nationwide Swedish case-control study found that, in general, bladder exstrophy is seen as an isolated malformation without additional, major malformations. Bladder exstrophy patients tended to be associated with a low birth weight (< 1,500 grams) and high maternal age (≥ 35 years).
The CASPR3 gene on chromosome 9 has been implicated by Boyadjiev and colleagues to be associated with the exstrophy complex. Another set of genes on the 9th chromosome has been identified to associate with bladder exstrophy. Genetic studies are attempting to determine where and if specific genetic factors can be found that are related to the exstrophy-epispadias complex. Multiple other possible gene loci have been identified but not confirmed.
Prenatal Diagnosis
Despite the magnitude of the defect in the lower abdominal wall and pelvic organ development, exstrophy of the bladder is still difficult to diagnose reliably by prenatal ultrasound. This is likely because of its rare incidence and that it is often mistaken for more common diagnoses of omphalocele or gastroschisis. Several groups have illustrated ultrasound findings important in the prenatal diagnosis of exstrophy. In a review of 25 prenatal ultrasounds with subsequent birth of a newborn with classic bladder exstrophy Gearhart et al described the main criteria for the prenatal diagnosis of exstrophy. These criteria included the absence of bladder filling, lower abdominal mass which becomes more protuberant as the pregnancy proceeds, a low-set umbilicus, separation of the pubic rami, and difficulties determining the sex of the baby.2,4,3,5 A more recent retrospective study out of Germany found the median gestational age was about 24 weeks. Again, all fetuses presented with the pathognomonic findings of nonvisualization of the fetal bladder and protruding abdominal mass below the umbilical cord insertion. In analyzing this data, bladder exstrophy should always be suspected on the basis of non-visualization of the bladder.
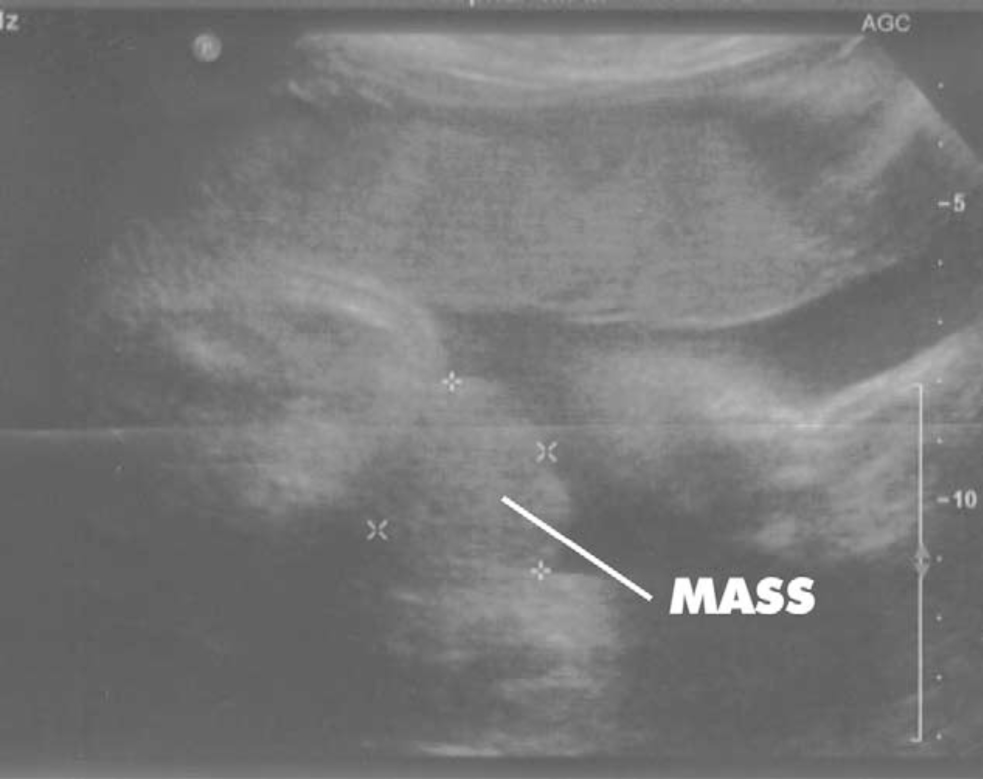
Figure 2 30 weeks gestation ultrasound demonstrating nonvisualization of the bladder and a lower abdominal mass.
It is felt that 3-D ultrasound and the increasing use of fetal MRI will improve the ability to diagnosis bladder and cloacal exstrophy. Prenatal diagnosis allows for prenatal counseling and arrangements to be made for delivery at a specialized exstrophy center. This allows for a multidisciplinary approach by teams with experience dealing with the unique nature of the exstrophy-epispadias complex. This includes availability of reconstructive teams in the immediate newborn period and psychosocial support for the parents and families. With fetal MRI, one may be able to distinguish between classic bladder exstrophy and cloacal exstrophy with MRI. This could aid in prenatal counseling for the mother.
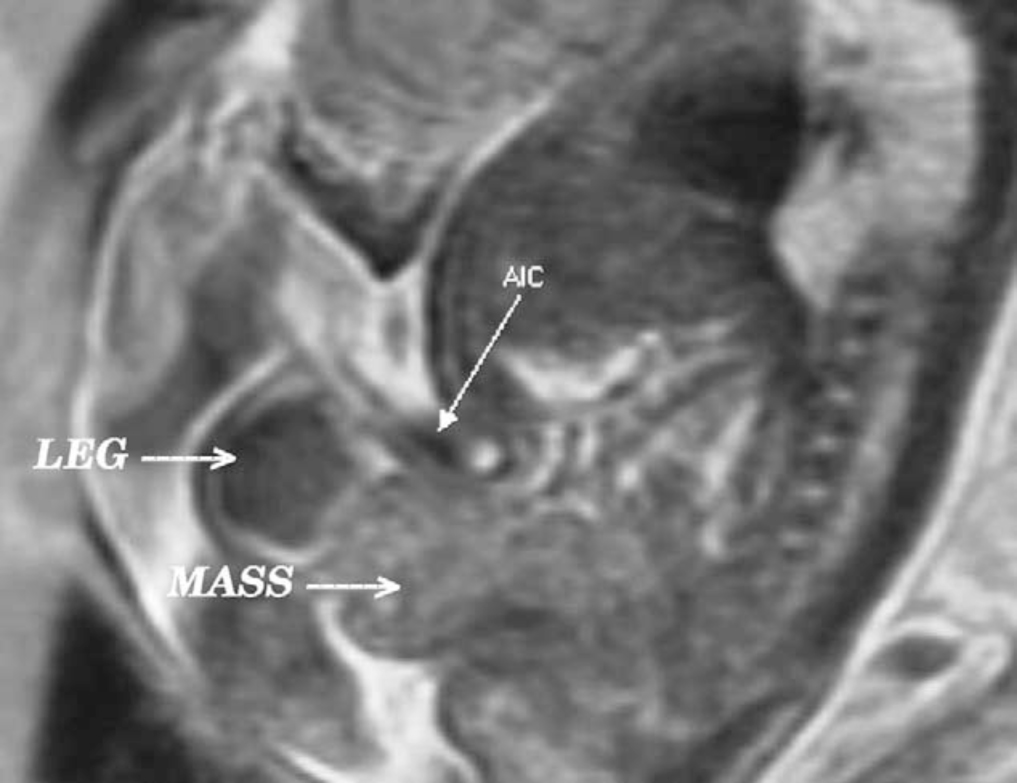
Figure 3 Sagittal view of bladder exstrophy (mass) below abdominal insertion of the umbilical cord (AIC) on T2 prenatal MRI.
The diagnosis of bladder exstrophy is made (or confirmed) at birth with visualization of the bladder plate characteristically protruding beneath the umbilical cord with divergent rectus muscles on either side leading to widely separated pubic bones.
Associated Anomalies
Skeletal Defects
The most obvious skeletal defect is the separation of the pubic bones, which is caused by the outward rotation of the innominate bones, eversion of the pubic rami, and a 30% shortage of bone in the pubic ramus.6 The mean external rotation of the posterior aspect of the pelvis was 12˚ on each side, retroversion of the acetabulum, and a mean 18˚ of external rotation of the anterior pelvis was determined by 3-D CT reconstructions. Further use of 3-D CT scans showed that the SI joint angle (before closure) was 10˚ larger in the exstrophy pelvis compared to age-matched controls and 10˚ more toward the coronal plane than sagittal. The bony pelvis was also 14.7˚ inferiorly rotated. The sacrum was 42.6% larger by volume measurements and had 23.5% more surface area. Combined, these deformities lead to a mean pubic diastasis of 4.2 cm at birth, increasing to 14.2 cm in adults. This compares to a symphysial width of 0.6 cm in control subjects. These deformities of the pelvic bones contribute to the shortened phallus, waddling gait, and outward rotation of the lower limbs in exstrophy patients. A study of 299 bladder exstrophy children indicated that spinal variations occur without clinical significance: spina bifida occulta, lumbarization or sacralization of vertebrae in 11%, uncomplicated scoliosis in 2.7%, and spinal dysraphism in 4%, including myelomeningocele, lipomeningocele, scimitar sacrum, and hemivertebrae. Only one patient demonstrated evidence of neurologic dysfunction.7
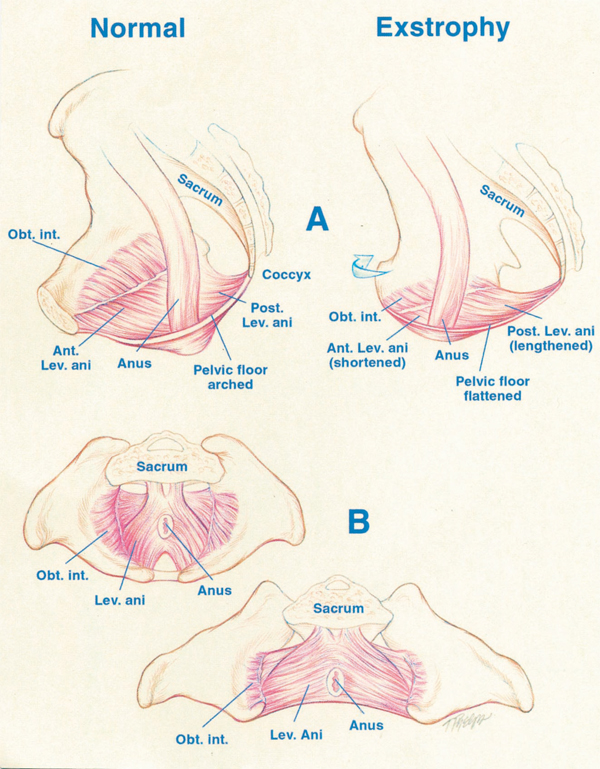
Figure 4 A, displacement of levator (Lev.) ani to more posterior (Post.) position in patient with exstrophy, that is 68% posterior to anus versus normal controls. Also note shortened anterior (Ant.) segment of levator ani in exstrophy 32% anterior to anus versus 48% in controls. Obt. int., obturator internus. B, greater outward rotation of 15.1˚ of obturator internus in exstrophy group versus controls. Also note that area encompassed by puborectalis is 2-fold that of controls and more flattened.
Pelvic Floor Defects
Data by Stec et al utilizing 3-D CT imaging demonstrated that the puborectal slings in classic bladder exstrophy patients support two times more body cavity than normal age-matched controls.4 While the levator muscle group in normal controls is evenly distributed posterior to anterior (52% to 48%) to the rectum, there is an uneven 68% to 32% posterior to anterior distribution in the exstrophy pelvis. There is also a significant flattening of the levators. A 31.7-degree decrease in the steepness is seen between the right and left halves of the levator ani and puborectalis sling. Consequently, the anus is anteriorly placed and sometimes patulous as a part of the posterior extent of the myofascial defect. These musculoskeletal malformations explain the increased rate of rectal prolapse, especially in the female exstrophy population.
Further study of the pelvic floor has been done with 3-D MRI and has impacted the understanding of the exstrophy pelvis for reconstruction. Williams et al demonstrated that the levator ani group was less dome shaped and more irregular in the exstrophy population prior to closure when compared to normal controls.8 There was also no relationship seen between the degree of pubic diastasis and the extent of disproportionate curvature of the levator ani muscle group . Review of post-closure pelvises by MRI revealed that in those with some degree of continence the intrasymphyseal distance was noted to be the shortest, the angle of the levator ani divergence sharpest, and the bladder neck most deeply positioned in the pelvis. Gargollo reported on MRI before and after exstrophy closure, noting that the puborectalis angle in those with dry intervals was decreased compared with that prior to closure.9 New reports of the use of 3D perineal ultrasound to evaluate the pelvic floor of adult exstrophy females showed that the ultrasound findings correlated well with MRI findings. These reports reinforce the necessity for aggressive dissection and posterior placement of the posterior urethra and bladder along with good reapproximation of the pubis at the time of closure. Osteotomies and pelvic fixation should be utilized if reconstruction is not done in the immediate newborn period.
Abdominal Wall Defects
There is a triangular defect because of the premature rupture of the abnormal cloacal membrane in the abdominal wall, and it is occupied by the exstrophy bladder and posterior urethra. This defect in the fascia is limited inferiorly by the intrasymphyseal band that represents the divergent urogenital diaphragm and connects the bladder neck and posterior urethra to the pubic rami. Wakim and Barbet investigated the relationship of the rectus muscle and fascia to the urogenital diaphragm and found no gross or histologic evidence of the striated sphincter.10 They did find evidence of bladder musculature extending laterally to the pubis where it interdigitates with fibers from the rectus fascia to form the fibrous urogenital diaphragm. The importance of radical incision of these fibers lateral to the urethral plate down to the level of the inferior pubic ramus and levator hiatus for the bladder and posterior urethra’s position deep in the pelvis were demonstrated by Gearhart and colleagues, using data from failed exstrophy closures where these fibers were intact at the time of reclosure.11
At the cephalad, limit of the triangular fascial defect is the umbilicus. The distance between the umbilicus and anus is foreshortened in bladder exstrophy because the umbilicus is well below the horizontal line of the iliac crest. Although an umbilical hernia is usually present, it is typically insignificant in size and repaired at the time of initial exstrophy closure.
Inguinal hernias are common. They are due to a lack of obliquity of the inguinal canal combined with large internal and external rings and persistence of the processus vaginalis. Connolly and associates reported an 81.8% incidence of inguinal hernia in males and 10.5% in females.12 It is recommended to explore the inguinal canals at the time of exstrophy closure and excise the hernia sac with repair of the transversalis fascia and muscular defect to prevent recurrence or a direct hernia regardless of staged approach or complete primary repair. In addition, in the setting of concomitant exstrophy closure and inguinal hernia repair, the inguinal hernia recurrence rate is more than 30 % (much higher in males), reflecting an innate weakness in the abdominal wall and lack of obliquity of the inguinal canal. This is especially prominent within the first 6 months following initial bladder closure by CPRE.
Anorectal Defects
The exstrophy patient’s perineum is short and broad and the anus directly behind the urogenital diaphragm. It is anteriorly displaced and corresponds to the posterior limit of the triangular fascia defect. The anal sphincter complex is also anteriorly displaced and should be preserved intact. These anatomic factors contribute to varying degrees of anal incontinence and rectal prolapse. Rectal prolapse frequently occurs in untreated exstrophy patients with a widely separated symphysis. Usually this is transient, easily reduced and disappears after bladder closure or cystectomy/urinary diversion. The appearance of prolapse of the rectum is also an indication to proceed with definitive management of the exstrophied bladder. If it occurs at any time after exstrophy closure, posterior urethra/bladder outlet obstruction should be suspected and immediate evaluation of the outlet tract by cystoscopy should be performed.
The implications of cloacal exstrophy (OEIS complex) upon the hindgut will be covered later in this chapter.
Male Genital Defects
The male genital defects are severe and challenging at the time of reconstruction. The phallus is short due to 50% deficiency in anterior corporal length with preservation in posterior length of the corporal body when compared to age-matched controls by MRI.3 The diameter of the posterior corporal segment was greater than in normal controls. The diastasis of the symphysis pubis increased the intrasymphyseal and intracorporeal distances, but the angle between the corpora cavernosa was unchanged because the corporal bodies were separated in a parallel fashion. This results in a penis that appears short because of the diastasis and the marked congenital deficiency of anterior corporal tissue. Releasing the dorsal chordee, lengthening the urethral groove, and mobilizing the crura in the midline to somewhat lengthen the penis can result in a functional and cosmetically appropriate phallus.
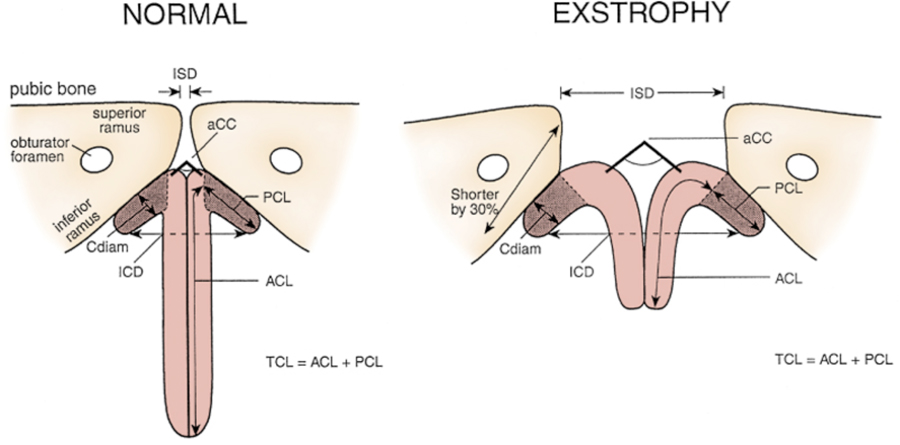
Figure 5 Penile and pelvic measurements in normal men and patients with exstrophy. ZSD, intersymphyseal distance. UCC, corpora cavernosa subtended angle. Cdiam, corpus cavernosum diameter. PCL, posterior corporeal length. ZCD, intercorporeal dlstance. ACL, anterior corporeal length. EL, total corporeal length
Epispadias is seen usually in conjunction with classic bladder exstrophy. However, rarely, it is seen as a solitary congenital defect in 1 in 117,000 males, and 1 in 150,000 to 300,000 females. It is characterized by the failure of the urethral plate to tubularize on the dorsum with the defect ranging from glanular to penopubic location. Most male patients also have some degree of penile dorsal chordee. Some female patients exhibit a bifid clitoris. In males, the likelihood of urinary incontinence can depend upon the severity of the epispadias defect. However, even those with glanular epispadias have been described has having incontinence due to “a field defect” impeding bladder and urethral contractility and coaptation. In contrast, almost all female epispadiac patients have urinary incontinence, given the high likelihood of a concomitant bladder neck defect.
Gearhart and associates used MRI to demonstrate in 13 adult men with bladder exstrophy that the volume, weight, and maximum cross-sectional area of the prostate appeared normal compared with published controls.13 However, they found that in none of the patients evaluated did the prostate extend circumferentially around the urethra, and the urethra was anterior to the prostate in all patients. The free and total PSA levels in adult bladder exstrophy men were found to be measurable but below the upper limits of established age-specific references ranges for normal men.13 There is a single report of a 56 year-old epispadias patient having prostate cancer with a PSA of 4.2 at the time of biopsy.14
The vas deferens and ejaculatory ducts are normal in the exstrophy patient as long as they are not injured iatrogenically during closure or reconstruction. The mean seminal vesicle length was found to be normal.
The autonomic nerves that innervate the corpus cavernosum (cavernous nerves) are displaced laterally in exstrophy patients, and these nerves are preserved in almost all exstrophy patients as potency is preserved after surgery. Retrograde ejaculation is found to occur after bladder closure and bladder neck reconstruction.
Testes frequently appear to be retractile but have adequate length in the spermatic cord to reach the flat, wide scrotum without need for orchiopexy. The testes have not been studied in a large group of postpubertal exstrophy patients but are generally believed to be not impaired. D’Hauwers et al reported the use of percutaneous sperm aspiration and intracytoplasmic sperm injection, and state in 3 exstrophy patients they had good success with sperm harvesting.(missing reference)
Female Genital Defects
In girls, the mons, clitoris, and labia are separated and the vaginal orifice is displaced anteriorly and stenotic. The clitoris is bifid and the vagina is shorter than normal controls but of normal caliber. The cervix is found on the anterior vaginal wall because the uterus enters the vagina superiorly. The fallopian tubes and ovaries are usually normal. The bifid clitoris should be reapproximated with the two ends of the labia minora to form a fourchette at the time of primary closure. Commonly, vaginal dilation or an episotomy may be required to allow satisfactory intercourse in the mature female. A study associated with the Association for the Bladder Exstrophy Community and social medial found 13- adult women with a history of bladder exstrophy. Of these, 28.5% were treated for uterine prolapse. 36.2 % reported pregnancies, and of these 68% reported complications with pregnancy.15 Most adult women require interventions, medical and surgical for gynecologic concerns post menarche.16
Yet, despite bladder exstrophy being a severe condition, healthy pregnancies are reported with long term consequences to continence and sexual health.17
Urinary Defects
The exposed bladder mucosa is susceptible to cystic or metaplastic changes and, therefore, must be irrigated frequently with saline and protected from surface trauma and exposure by a protective membrane until surgical bladder closure can be performed. Commonly plastic wrap (i.e., Saran Wrap) is sufficient. The mucosa at birth can have a segment of ectopic bowel mucosa, an isolated bowel loop, or most commonly hamartomatous polyps.
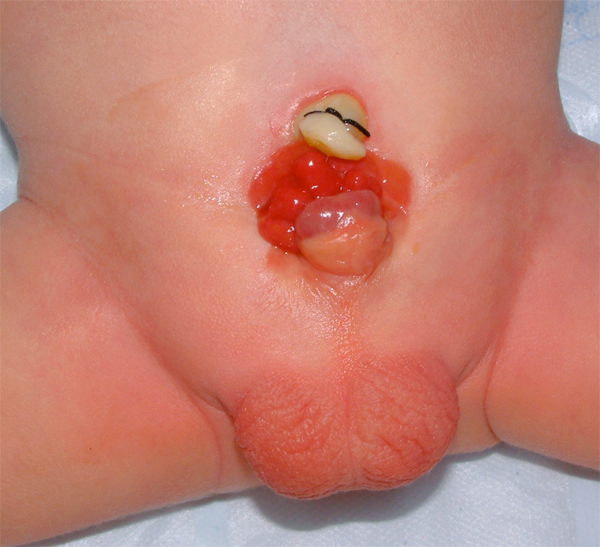
Figure 6 Newborn with classic bladder exstrophy. Note multiple polyps on bladder plate.
Shapiro and colleagues characterized the neuromuscular function of the bladder. They showed that muscarinic cholinergic receptor density and binding affinity were similar in exstrophy and control subjects.18 Bladder biopsies from 12 newborns with bladder exstrophy, compared to age-matched controls, found an increase in the ratio of collagen to smooth muscle in the exstrophy bladders.19 The type of collagens was analyzed and a normal distribution of type I collagen was present, but a threefold increase in type III collagen was found. It was later seen that those patients who demonstrated bladder growth by measuring capacity after successful closure, who were free of infection had a markedly decreased ratio of collagen to smooth muscle. Primary cultures of exstrophy bladder smooth muscle cells were shown to have growth characteristics similar to those previously reported in non-exstrophy cells, showing that they likely retain their potential for growth and function. Mathews and associates found that the average number of myelinated small nerve fibers per field was significantly reduced in the exstrophy bladders compared with controls.20 Preservation of larger nerve fibers was seen, which led the study to hypothesize that bladder exstrophy in a newborn represented an earlier stage of bladder development. Multiple immunocytochemical and histochemical markers, vasoactive intestinal polypeptide (VIP), neuropeptide Y (NPY), substance P (SP), calcitonin gene-related product (CGRP), protein gene product (PGP) 9.5, and nicotinamide adenine dinucleotide phosphate diaphorase (NADPHd) have been studied, and there was no evidence of bladder muscle dysinnervation morphologically in any cases of bladder exstrophy. However, cases of bladder exstrophy after failed reconstruction did have muscle innervation deficiencies that increased subepithelial and intraepithelial innervations. Microarray analysis of exstrophic bladder smooth muscle compared to ‘healthy’ controls showed what appears to be a developmentally immature finding in the exstrophy bladder smooth muscle.Therefore, it is felt that although the bladder in an exstrophy patient may be immature it has the potential for normal development after a successful initial closure.
Bladder plate polyps were found to be two types with overlapping findings: fibrotic and edematous. Both were associated with overlying squamous metaplasia in 50% of cases. Varying degrees of von Brunn’s nests, cystitis cystica and cystitis glandularis were noted. Cystitis glandularis was noted in a higher percentage of secondary closures. Future surveillance of those cystisis glandularis patients is recommended given their potential risk for adenocarcinoma. This can be done with urine cytology and cystoscopy as they enter adulthood.21
The bladder plate may invaginate or bulge through a small fascial defect at birth but the true estimate of the bladder plate cannot be evaluated completely until the newborn is under anesthesia and fully relaxed. A small, fibrosed, inelastic bladder and/or one that is covered with polyps may make a functional repair challenging and potentially impossible.
Normal cystometrograms were obtained in 70–90% of those assessed in a group of continent exstrophy patients with normal reflexive bladders.22 An evaluation of 30 exstrophy patients at various phases of modern staged repair prior to bladder neck reconstruction found 80% to have compliant and stable bladders after bladder neck reconstruction. Approximately half maintained normal bladder compliance and fewer maintained normal stability. It was felt by the authors of that study that 25% of exstrophy patients might maintain normal detrusor function after reconstruction.23 The microstructure of the bladder of exstrophy patients at various points in modern staged repair was found to have different caveoli (important intracellular structures for cell-cell signaling). These caveoli were felt to be normal in those with a successful closure and improving bladder capacity but lacking in those who required an augmentation cystoplasty. They noted that the ultrastructure of cells also was abnormal in the group that failed initial closure.
The remainder of the urinary tract is usually normal, but anomalies do occur. Horseshoe kidney, pelvic kidney, hypoplastic kidney, solitary kidney, and dysplasia with megaureters can all be encountered. The course of the ureter is abnormal in terms of termination. Because the peritoneal pouch of Douglas, between the bladder and the rectum, is enlarged and unusually deep, the ureter is forced down laterally in its course across the true pelvis. The distal segment approaches the bladder inferiorly and laterally to the orifice. This results in vesicoureteral reflux in 100% of exstrophy cases. Ureteral reimplantations are done at the time of bladder neck repair but sometimes are needed sooner. If there are problems with infections and excessive outlet resistance, ureteral reimplantation may be needed prior to bladder neck reconstruction or at any point if severe reflux and upper tract issues develop.
Evaluation and Management at Birth
In the delivery room the umbilical cord should be tied with 2-0 silk close to the abdominal wall, so the umbilical clamp does not irritate or traumatize the exposed bladder mucosa. The bladder mucosa should be frequently irrigated with warm saline and always covered with a protective clear plastic wrap until the time of closure. The bladder should be irrigated, and plastic wrap changed at each diaper change.
A multidisciplinary approach is important. The team should include, but not be limited to, a pediatric urologist, pediatric orthopedic surgeon, pediatric anesthesiologist, neonatologist, pediatric psychiatrist (with expertise and experience in genital anomalies) and social workers. Studies have proven that the parents of exstrophy patients experience a significant amount of stress. The parents’ stress should not be overlooked during the initial and long-term care of the patient.24 The parents should be reassured that children with classic bladder exstrophy are generally healthy, robust infants with the prospect of leading a very normal life. Effective reconstruction to allow urinary storage, drainage, and control can be expected with acceptable cosmetic appearance. The support of psychologists, nurses, and parents of other children with exstrophy is invaluable.
A neonatologist should evaluate the patient from a general pediatric and cardiopulmonary standpoint with the likelihood of major surgery in the first 48 hours of life. A cardiac echo is commonly done to rule out significant cardiopulmonary anomalies that would preclude early reconstruction. A renal ultrasound should be obtained to evaluate the upper urinary tracts. A KUB is done to evaluate the pelvis bony anatomy and a spinal ultrasound to rule out an associated spinal dysraphism.
It is essential that a pediatric genitourinary surgeon with experience and interest in the exstrophy-epispadias complex evaluate the newborn exstrophy patient, as the impact of a major birth defect is significantly worse by inappropriate initial management.25,26,27
In those patients with ambiguous genitalia in addition to bladder exstrophy the parents should be educated and counseled by a multidisciplinary disorders of sexual differentiation team but should understand that the need to change the gender rearing in classic bladder exstrophy is almost nonexistent in the male infant with current reconstructive outcomes.
As many of these cases still go undetected until the time of delivery, most will require transport to an exstrophy center soon after birth. During travel, the bladder should be protected by a clear plastic membrane and kept moist to protect the delicate bladder mucosa.
Surgical Reconstruction
The goals of surgical reconstruction in the exstrophy patient are to correct the urogenital defects providing a reservoir that is adequate for urinary storage at low pressures with the ability to empty completely without compromising renal function, to create functional and cosmetically acceptable external genitalia, and to maximize patient quality of life.
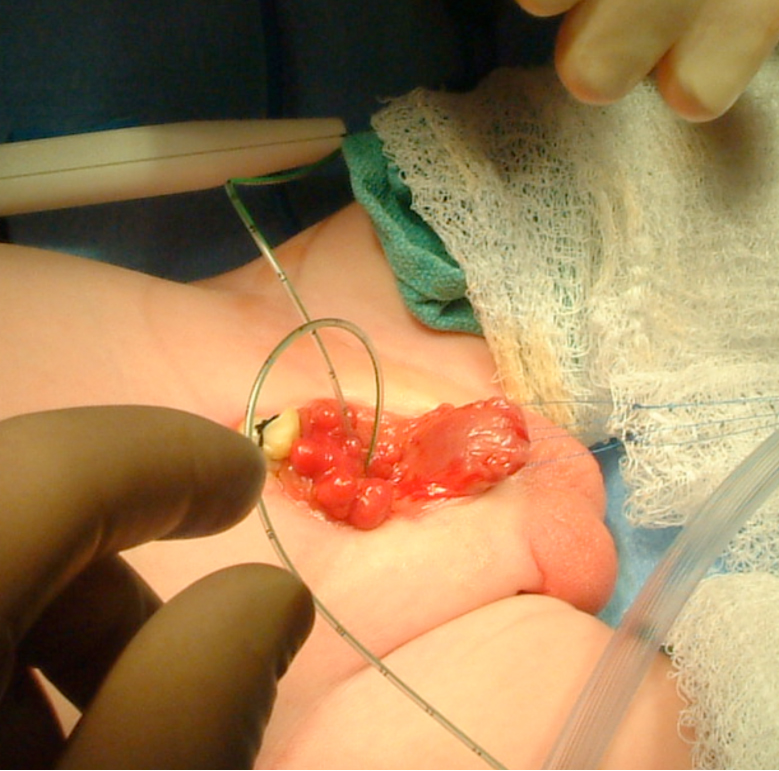
Figure 7 Intubation of bilateral ureteral orifices in preparation for dissection.
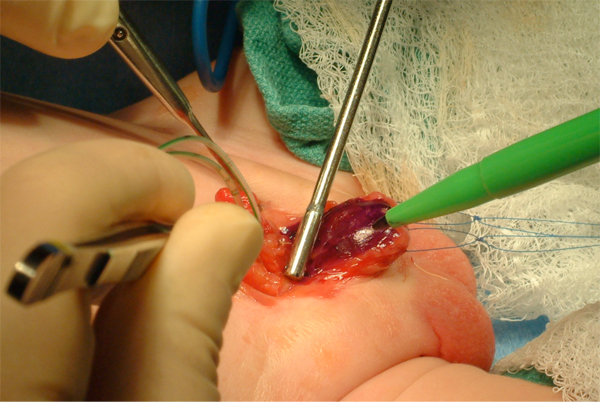
Figure 8 Marking of urethral plate. Notice two traction sutures on each hemiglans.
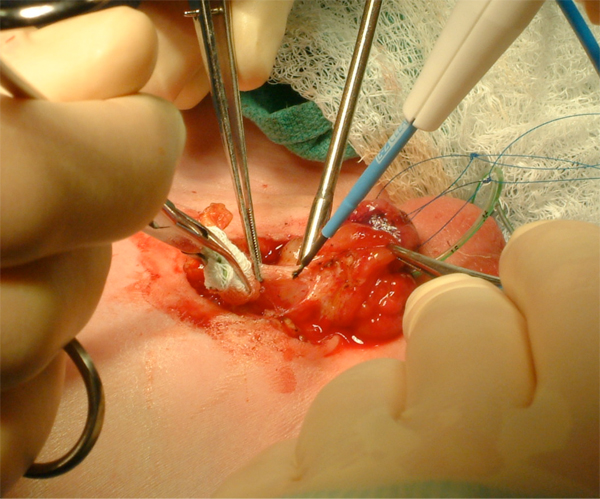
Figure 9 Aggressive bladder mobilization, including the umbilicus.
Early attempts at bladder exstrophy reconstruction were unsuccessful and patients had short life expectancies. Therefore, for many years the management for exstrophy consisted of removal of the exstrophic bladder and urinary diversion commonly by ureterosigmoidostomy. Various staged repairs began to show early success in the 1950s.In the 1970s, the preliminary constructs of staged repair that are utilized today were initiated. This developed into the modern staged repair of exstrophy that is commonly used today.28 In the late 1980s an anatomical approach to exstrophy repair began and has been modified into what is now referred to as the complete primary repair of exstrophy or Mitchell technique.29 Currently, most patients are managed either with a complete primary repair of exstrophy (CPRE) or modern staged reconstruction of exstrophy (MSRE).28 A great deal of discussion continues regarding the optimal treatment of exstrophy in the newborn period. With that in mind, ureterosigmoidostomy remains a popular and preferred reconstruction in many parts of the world because it reliably achieves urinary continence and is relatively safe for those without access to dependable health care facilities or a specialized exstrophy center.
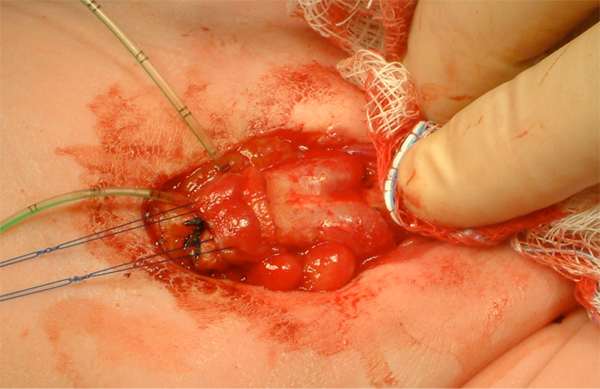
Figure 10 Identification of bilateral corporal bodies after degloving of phallus.
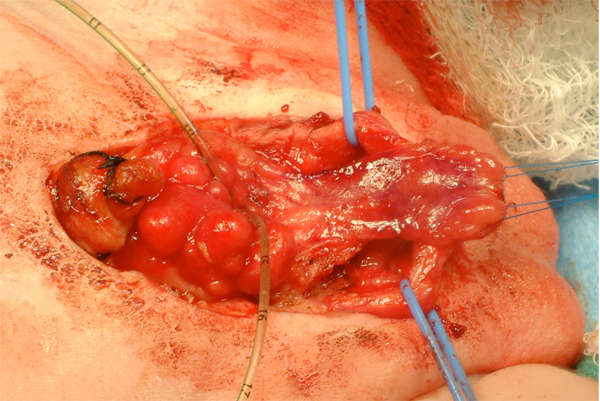
Figure 11 After separation of the corporal bodies and mobilization of the urethral plate.
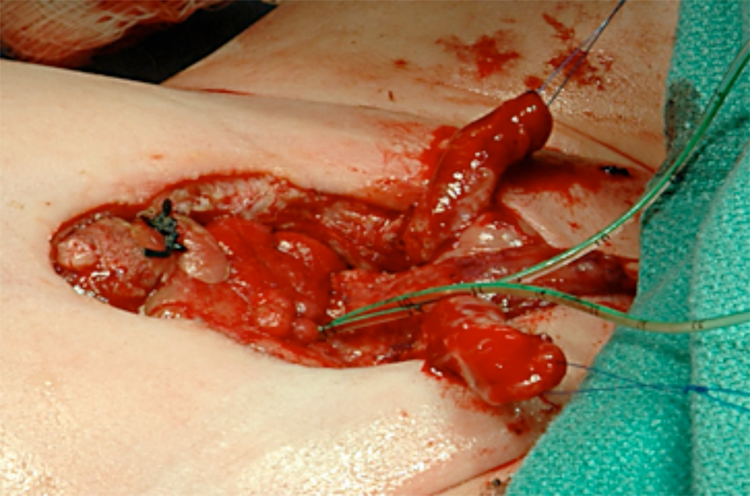
Figure 12 After complete separation of the corporal bodies and glans.
Complete Primary Repair of Exstrophy
The CPRE is the most recent development in surgical management of exstrophy patients pioneered by Dr. Mitchell in 1989.30 This approach has been adopted and reproduced by multiple centers of excellence for exstrophy management. In the CPRE, an anatomic approach to reconstruction includes bladder closure with bladder neck remodeling and a disassembly technique for epispadias repair with or without osteotomies in one setting. It is felt this allows for bladder cycling and more ‘normal’ growth and development because the bladder experiences an outlet resistance. The disassembly technique for epispadias repair also allows for division of the intersymphyseal ligaments and appropriate anatomic placement of the bladder neck and posterior urethra deep into the pelvis in its orthotopic position.When done within the first 72 hours of life, the pelvis is malleable enough to close without osteotomies. The patients still necessitate Bryant’s traction for approximately 1 week and then lower extremity casting for 3 weeks to prevent tension on the pelvis closure. The incidence of dehiscence and bladder prolapse is rare in the modern era.
The CPRE is advocated as an approach to allow for maximal development of the bladder and define those patients whose bladders will grow and develop earlier than those managed in a staged manner. Normalizing the anatomy at the initial closure just after birth may have other benefits such as minimizing the family’s psychosocial trauma. Patients whose bladder does develop will likely undergo fewer procedures than in a staged fashion.
Continence has been reported after CPRE to be 76%, defined as dry intervals longer than 2 hours and spontaneous voiding without catheterizations. However, a significant percentage of patients will likely still require a formal bladder neck procedure to achieve continence. In another series, 75% of patients after CPRE were continent with intervals of 4 hours and dry at night with 31.3% requiring CIC.
Concerns with the CPRE are related to potential for renal deterioration related to a high pressure lower urinary system, risk of penile injury or loss with disassembly at such a young age, and requirement for multiple procedures despite the name ‘complete repair.’ Close follow-up of the upper tracts is important in exstrophy regardless of repair. Hydronephrosis, pyelonephritis and renal scarring are seen after CPRE and should be managed aggressively with prophylactic antibiotics since vesicoureteral reflux is expected post operatively. Long-term follow-up in the Seattle series shows that mild (45%), moderate (17.8%), and severe (7.1%) hydronephrosis is seen after CPRE. But half of those with hydronephrosis, and in all with severe hydronephrosis, it was transient. Borer and associates reported an incidence of pyelonephritis of 28% and DMSA renal scaring of 19% after CPRE.31 The need for bilateral ureteral reimplantation after CPRE exclusive of those done at the time of bladder neck repair are seen to be 25–34%.31,32,33
Approximately 36–68% of patients will be left with a hypospadias after CPRE that will require further penile surgery.30,34,31,32,33 Glans necrosis, penile skin loss and/or penile tissue loss has been reported after CPRE but is quite rare when performed at an exstrophy centers of excellence.
A recent systematic review examined the results of ten groups using the CPRE technique with 236 patients. Hypospadias repair was required for most boys having complete penile disassembly (22.7–68 %). Ureteral reimplantation was required as a separate procedure in 48–66%. Most children eventually also required bladder neck reconstruction for continence. Overall, voiding, without BNR was noted in 16–37 % of children in the reported series.
Comparing the number of procedures that a patient may be expected to undergo is a controversial topic when comparing the CPRE and MSRE. Reports vary widely about the number and types of procedures and the reports are fraught with potentials for bias making it difficult to draw true conclusions on this subject. However, it is accepted that patients managed in a CPRE or MSRE approach will likely require multiple procedures and the outcomes are better if managed at a center of excellence for exstrophy.
In 2013, the Multi-Institutional Bladder Exstrophy Consortium (MIBEC) was established to refine the CPRE, decrease complications, and improve outcomes. Since that time, MIBEC has managed to pool their outcomes (both before and after the consortium) in order to better study this relatively rare disorder, and critically evaluate their technique. Through the consortium, pediatric urologists work collaboratively as “surgical coaches” at the host institution to compound multiple experts’ expertise.
Other groups have adopted this collaborative approach for the surgical treatment of bladder exstrophy. For example, the Pediatric Urology Midwest Alliance (PUMA) pooled their outcomes to assess outcomes of bladder augmentation/diversion and need for clean intermittent catheterization.
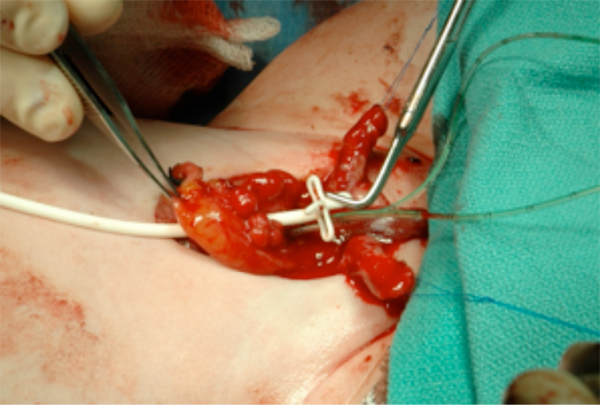
Figure 13 Placement of suprapubic tube prior to bladder closure. This will exit the abdomen at the umbilicus.
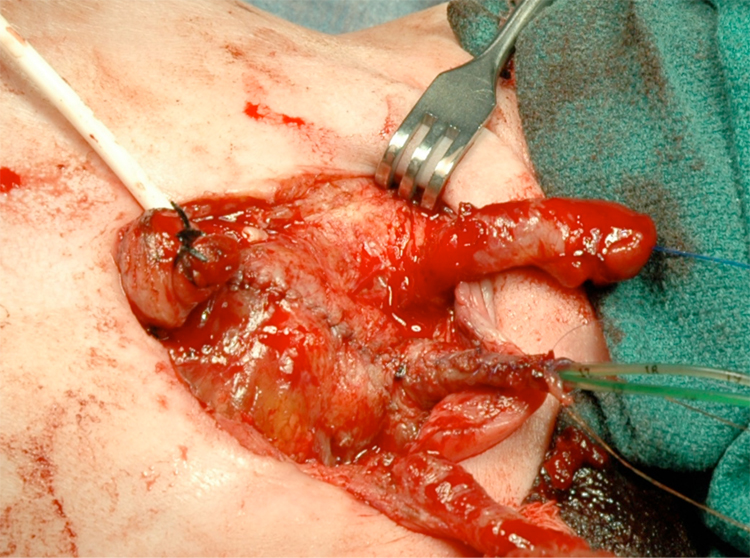
Figure 14 Bladder and urethra closed in 2 layers as a single unit. Note bilateral ureteral catheters exiting via the urethral meatus.
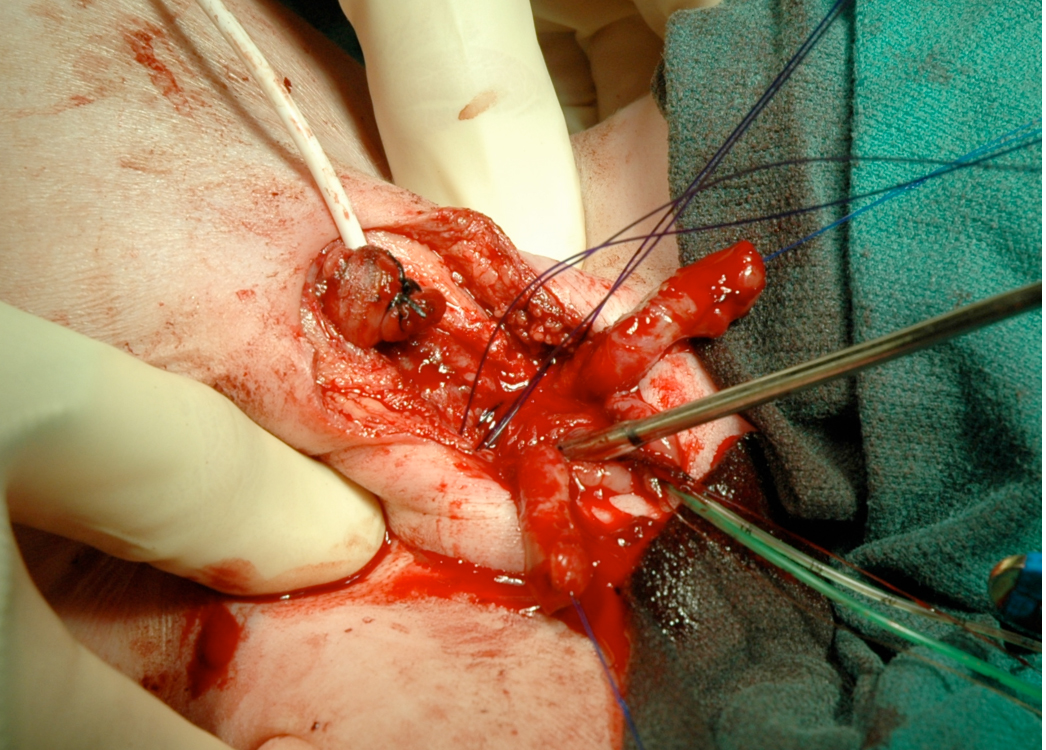
Figure 15 Internal rotation of the pelvis to reapproximate the pubis when reconstructed in the first 72 hours of life can be accomplished.
Modern Staged Reconstruction of Exstrophy
The MSRE as now practiced has evolved from the original work by Cendron and Jeffs.25,26 The Hopkins group, led by Dr. Gearhart, currently has the largest exstrophy population and writes the majority of the literature regarding the MSRE. In the MSRE, the goal at initial reconstruction is to convert the exstrophic bladder to a complete epispadias. Pelvic osteotomies are performed in conjunction with bladder closure when indicated. It is felt that this will allow for protection from renal dysfunction because the patient is still incontinent but can also stimulate bladder growth since there is now some bladder outlet resistance. The epispadias repair is performed between 6-12 months of age and testosterone stimulation is provided preoperatively. The bladder neck repair for continence is performed between 4-5 years of age if they have an adequate bladder capacity and are determined to be ready to participate in a postoperative voiding program.35 If these criteria are not met, the patient is left incontinent until the bladder grows and maturity improves or they are diverted with an augmentation with a catheterizable channel and bladder neck closure when necessary.
Continence rates after MSRE are reported by Gearhart in males to be 70% and females to be 74% with dry periods greater than 3 hours with spontaneous voiding and dry at night without CIC.35 They have concluded that a bladder capacity of 100 ml predicts success at the time of bladder neck reconstruction utilizing the modified Young-Dees-Leadbetter repair. This increased the chance of continence and time to achieve continence in their reviews.35,36 If this 100 ml capacity is not achieved it is felt they should undergo an augmentation at the time of reconstruction for continence. They also found that females were more likely to achieve continence and at a shorter duration after bladder neck reconstruction.36 Their reports also show similarly low complication rates. In males after bladder closure, epispadias repair, and bladder neck reconstruction the total complication rate was 41.7% with one incidence of primary closure failure.35 In females after bladder closure and bladder neck reconstruction the total complication rate was 19.5%.36 In their modern series if successful bladder growth occurs 19.4% of males and 17% of females failed bladder neck reconstruction and have undergone or will require an augmentation with a catheterizable stoma and bladder neck closure as indicated.35,36
In the MSRE, if a patient’s bladder capacity does not develop to the level that predicts success with bladder neck reconstruction, the patient is moved directly to an augmentation for continence. This is reasonable but clouds comparisons between MSRE and CPRE. The MSRE reports those that complete the series. Because the CPRE performs a continence procedure at the initial closure, they are including those patients whose bladders may not have developed and never been a candidate for a bladder neck reconstruction had they been managed by MSRE. Also, it is difficult to determine how many, if any, of those bladders that did not develop to 100 ml capacity by continence age would have grown had they had a CPRE at initial closure with increased outlet resistance available to promote development. There also may be a certain subset of exstrophy bladders that are ‘bad’ and will not grow regardless of closure technique but are unable to be identified at birth. These factors make it difficult to define success and failure when comparing techniques. Further long term prospective analysis needs to continue in an unbiased and open minded fashion to promote the interchange of ideas that can hopefully lead to the next breakthroughs in management of exstrophy that will continue to improve the quality of life of these patients.
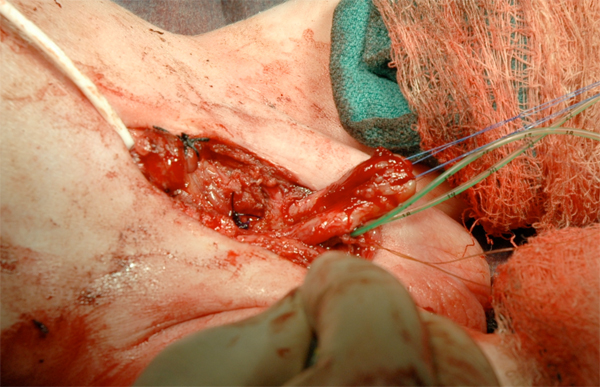
Figure 16 Phallus reconstruction.
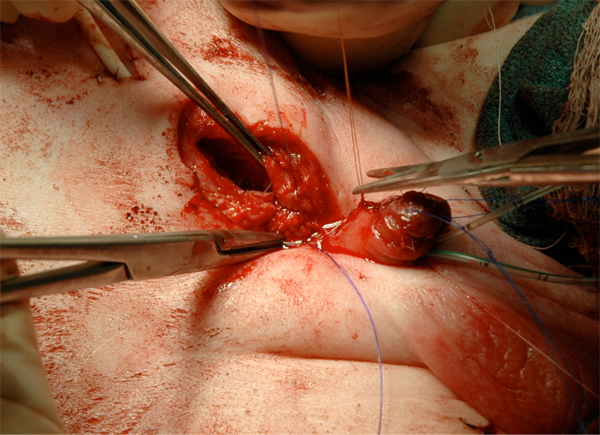
Figure 17 Fascial closure.
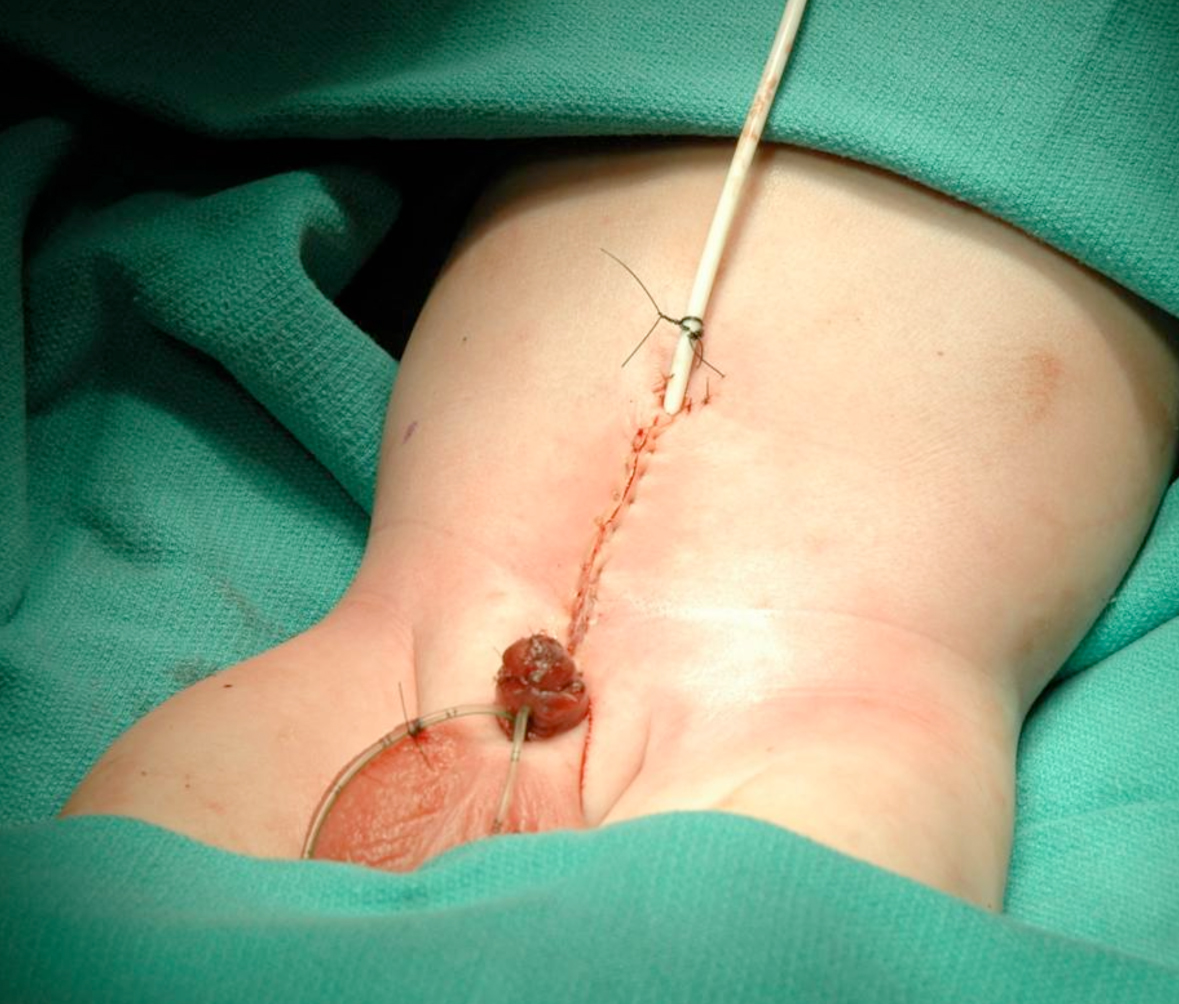
Figure 18 Abdominal wall closure.
Summary of Agreed upon Surgical Reconstruction Tenants
- Management should be done at an exstrophy center of excellence and with a multidisciplinary team with the experience and interest necessary to care for these patients long-term.
- Bladder plate should be moist and covered with saran wrap after birth until closure
- Early successful primary closure is paramount to achieve continence later
- Osteotomies are necessary if pelvis is closed at > 72 hours of life
- Close follow-up of upper tracts is important after bladder closure
- Multiple procedures are required for to achieve continence and reconstruction of anatomy
- Augmentation with or without catheterizable stoma and bladder neck closure may be required in those who fail to achieve continence or a > 100 mL capacity bladder prior to bladder neck reconstruction.
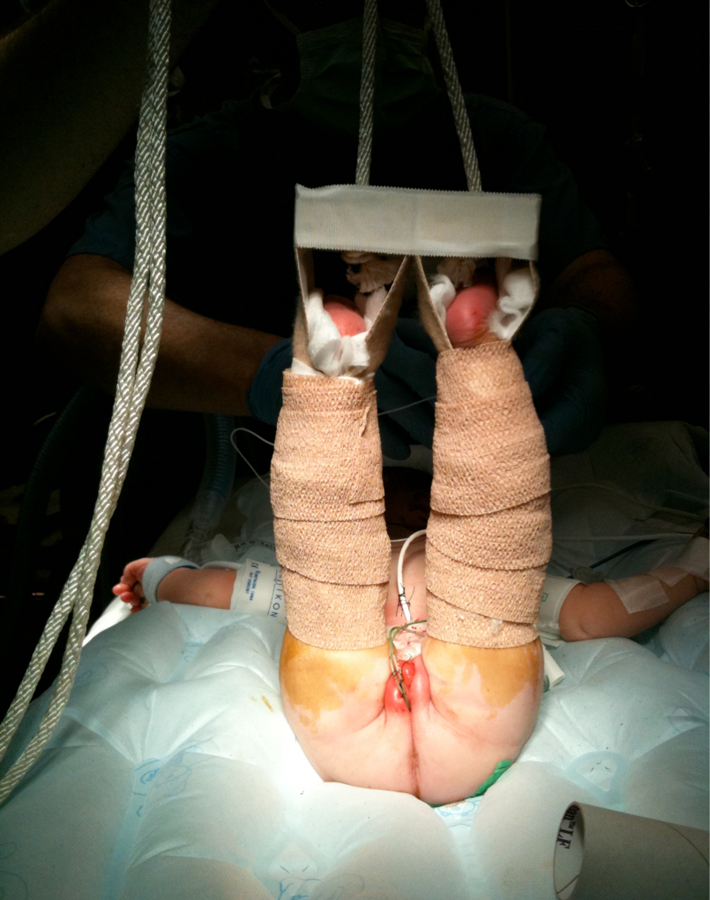
Figure 19 Modified Bryant’s traction.
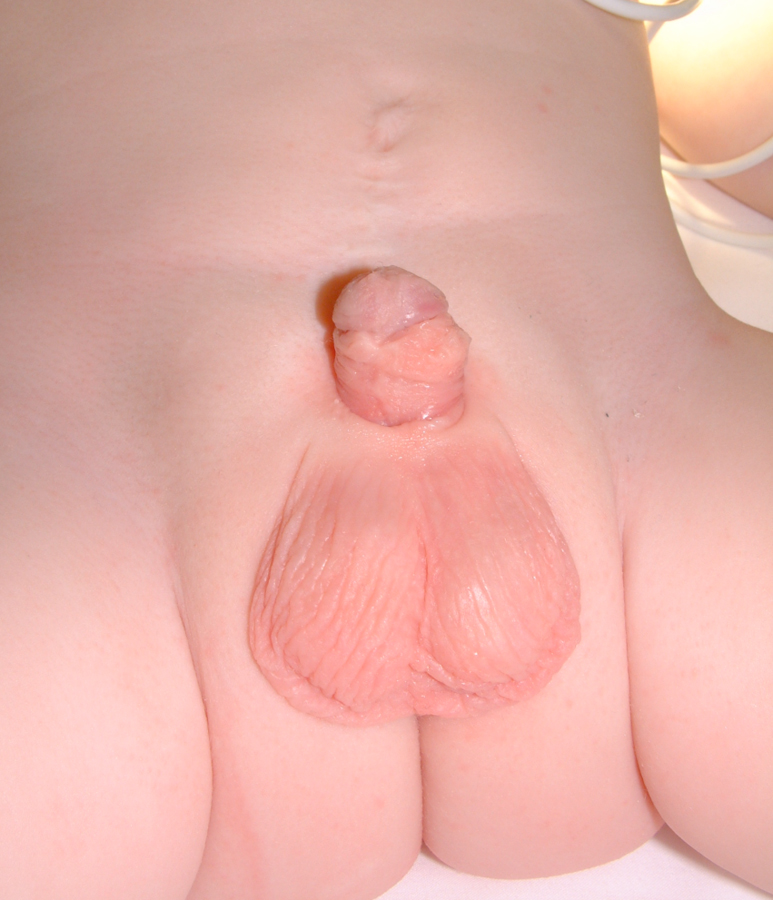
Figure 20 Postoperative.
Sexual Function in the Exstrophy Patient
There are reports of adult male exstrophy patients fathering or initiating a pregnancy. This shows that fertility is possible in these patients, although it may not be commonplace. Shapiro’s large series of 2,500 patients only documented 38 males who had fathered children.3 Semen analyses studies comparing men who underwent primary repair to ureterosigmoidostomy diversion found a normal sperm count in only one of eight in the closure group and in four of eight in the diverted group. These differences were attributed to retrograde ejaculation and iatrogenic injury during functional closure of the bladder. Another study found that none of their reconstructed patients could ejaculate normally, nor had they fathered children. Five patients who had not undergone reconstruction had normal ejaculation and two had fathered children.This leads to the conclusion that the male patient is at high risk of infertility after reconstruction. The use of assisted reproduction in any fashion has been shown by Bastuba and coworkers to be successful in 13 males with exstrophy that led to successful pregnancy with no incidence of exstrophy in the offspring.37
Woodhouse reported that the sexual function and libido of the exstrophy patient is normal.38 Multiple reports show that erectile function is maintained in a vast majority after urethral and phallic reconstruction, and that ejaculation is often not normal but is usually present. Most reported satisfactory orgasms, and described intimate relationships as serious and long-term.39 However, a recent study using an anonymous social media survey to those men in the Association for the Bladder Exstrophy Communities report the Penile Perception Score, International Index of Erectile Function to be lower than published controls. Interestingly, a higher Penile Perception Score correlated with a higher IIEF-15 (sexual satisfaction) score. Recent studies by Rubenwolf et al demonstrate that the sexual satisfaction of both adult males and females status post a continent diversion appears to be comparable to that of those in whom that bladder is preserved with the majority in stable relationships.40
The female external genitalia are now routinely fully reconstructed at the time of exstrophy closure. Previously this reconstruction consisted of a cosmetic reapproximation of the bifid clitoris and anterior labia to make a fourchette but did not address the inherent anatomic abnormality of the vagina’s location and angle relative to the abdomen and perineum in exstrophy females. Further reconstruction during adolescent years, prior to initiation of sexual activity or use of tampons in some female exstrophy patients was not uncommon. An application of the total urogenital complex mobilization was applied and resulted in successful correction of the location and angle of the vagina in female exstrophy patients.18 Successful intercourse has been reported in all patients in one study and dyspareunia was reported in a minority.19 A large series also reported that female exstrophy patients greater than 18 years of age had normal sexual desires and many were sexually active with normal orgasms. Some patients were self-conscious of and limited their sexual activity because of the cosmetic appearance of their external genitalia. A monsplasty is an important part of reconstruction in females and use of hair bearing skin and fat to cover the midline defect is routine. A later repair with the use of rhomboid flaps was reported with good success.41
Obstetric and Gynecologic Implications
Many women with bladder exstrophy have successfully delivered normal offspring (45 women with 49 children in one report).Another study showed 40 women, ages 19 to 36 that were treated for bladder exstrophy as infants, and out of those 40 women 14 pregnancies were reported in 11 women. Out of those 14 pregnancies were 9 normal deliveries, 3 spontaneous abortions, and 2 elective abortions. Uterine prolapse occurred in 7 of the 11 patients during pregnancy. It is seen to be very common for these women to have cervical and uterine prolapse after pregnancy and delivery.42 In these early reports, all women apparently had undergone prior permanent urinary diversions but recent reports show that successful pregnancies have been reported in women who have undergone continent urinary diversions.Cesarean sections were performed in women with functional bladder closures to eliminate stress on the pelvic floor and to avoid traumatic injury to the urinary sphincter mechanism.42
As mentioned earlier, pelvic organ prolapse appears to be a significant problem in female exstrophy patients. It is commonly seen during and after pregnancy or delivery, possibly in up to half of patients.19 It can occur at young ages and without prior sexual activity or pregnancy.19,43 The anterior displacement of the vaginal os and marked posterior displacement of the dorsorectalis sling and its deficient anterior compartment were theorized as reasons for significant findings of prolapse.44 It is felt that more modern reconstruction of the pelvic floor and anatomical replacement of the bladder into the pelvis and use of osteotomies may improve this troubling problem. Previous reports showed that uterine suspension was only modestly successful in prevention of recurrent prolapse.44 However, Stein reported that uterine fixation by sacrocolpopexy corrected prolapse in 13 females with greater than 25 years of follow-up.
References
- Hsieh K, O’Loughlin MT, Ferrer FA. Bladder exstrophy and phenotypic gender determination on fetal magnetic resonance imaging. Urology 2005; 65 (5): 998–999. DOI: 10.1016/j.urology.2004.12.060.
- Stec AA, Pannu HK, Tadros YE, Sponseller PD, Fishman EK, Gearhart JP. Pelvic Floor Anatomy In Classic Bladder Exstrophy Using 3-dimensional Computerized Tomography: J Urol 2001; 166 (1444): 1444–1449. DOI: 10.1097/00005392-200110000-00066.
- Silver RI, Partin AW, Epstein JI, Chan DW, Carter HB, Jeffs RD, et al.. Prostate-specific antigen in men born with bladder exstrophy. Urology 1997; 49 (2): 253–256. DOI: 10.1016/s0090-4295(96)00438-4.
- Purves JT, Gearhart JP. The Bladder Exstrophy–epispadias–cloacal Exstrophy Complex. J Pediatr Urol 1999; 22 (8): 386–415. DOI: 10.1016/b978-1-4160-3204-5.00030-x.
- Schober JM, Carmichael PA, Hines M, Ransley PG. The Ultimate Challenge Of Cloacal Exstrophy. J Urol 2002; 167 (300): 300–304. DOI: 10.1097/00005392-200201000-00088.
- Lee RS, Grady R, Joyner B, Casale P, Mitchell M. Can a Complete Primary Repair Approach be Applied to Cloacal Exstrophy? J Urol 2006; 176 (6): 2643–2648. DOI: 10.1016/j.juro.2006.08.052.
- Ben-Chaim J, Peppas DS, Sponseller PD, Jeffs RD, Gearhart JP. Applications of Osteotomy in the Cloacal Exstrophy Patient. J Urol 1995; 5 (1): 865–867. DOI: 10.1097/00005392-199508000-00146.
- Silver RI, Sponseller PD, Gearhart JP. Staged Closure Of The Pelvis In Cloacal Exstrophy: First Description Of A New Approach. J Urol 1999; 161 (1): 263–266. DOI: 10.1016/s0022-5347(01)62128-1.
- Adams MC, Mitchell ME, Rink RC. Gastrocystoplasty: An Alternative Solution to the Problem of Urological Reconstruction in the Severely Compromised Patient. J Urol 1988; 140 (5 Part 2): 1152–1156. DOI: 10.1016/s0022-5347(17)41986-0.
- Hendren WH. Ileal Nipple for Continence in Cloacal Exstrophy. J Urol 1992; 148 (2 Part 1): 372–379. DOI: 10.1016/s0022-5347(17)36601-6.
- Tank ES, Lindenauer SM. Principles of management of exstrophy of the cloaca. Am J Surg 1970; 119 (1): 95–98. DOI: 10.1016/0002-9610(70)90018-8.
- Gearhart JP, Jeffs RD. Techniques to Create Urinary Continence in the Cloacal Exstrophy Patient. J Urol 1991; 146 (2 Part 2): 616–618. DOI: 10.1016/s0022-5347(17)37871-0.
- Ricketts RR, Woodard JR, Zwiren GT, Andrews HG, Broecker BH. Modern treatment of cloacal exstrophy. J Pediatr Surg 1991; 26 (4): 444–450. DOI: 10.1016/0022-3468(91)90993-4.
- Austin PF, Homsy YL, Gearhart JP, Porter K, Guidi C, Madsen K, et al.. The Prenatal Diagnosis Of Cloacal Exstrophy. J Urol 1998; 160 (1179): 1179–1181. DOI: 10.1097/00005392-199809020-00061.
- Hamada H, Takano K, Shiina H, Sakai T, Sohda S, Kubo T. New Ultrasonographic Criterion For The Prenatal Diagnosis Of Cloacal Exstrophy: Elephant Trunk-like Image. J Urol 1999; 162 (6): 2123–2124. DOI: 10.1016/s0022-5347(05)68138-4.
- Husmann DA, McLorie GA, Churchill BM. Phallic Reconstruction in Cloacae Exstrophy. J Urol 1989; 142 (2 Part 2): 563–564. DOI: 10.1016/s0022-5347(17)38816-x.
- Mathews, Perlman, Marsh, Gearhart. Gonadal morphology in cloacal exstrophy: implications in gender assignment. BJU Int 1999; 84 (1): 99–100. DOI: 10.1046/j.1464-410x.1999.00148.x.
- Reiner WG. Psychosexual development in genetic males assigned female: the cloacal exstrophy experience. Child Adolesc Psychiatr Clin N Am 2004; 13 (3): 657–674. DOI: 10.1016/j.chc.2004.02.009.
- Baker Towell DM, Towell AD. A Preliminary Investigation Into Quality of Life, Psychological Distress and Social Competence in Children With Cloacal Exstrophy. J Urol 2003; 169 (5): 1850–1853. DOI: 10.1097/01.ju.0000062480.01456.34.
- Taghizadeh A, Qteishat A, Cuckow PM. Restoring Hindgut Continuity in Cloacal Exstrophy: A Valuable Method of Optimising Bowel Length. Eur J Pediatr Surg 2009; 19 (03): 141–144. DOI: 10.1055/s-0029-1192048.
- Husmann DA, McLorie GA, Churchill BM. Closure of the Exstrophic Bladder: An Evaluation of the Factors Leading to its Success and its Importance on Urinary Continence. J Urol 1989; 142 (2 Part 2): 522–524. DOI: 10.1016/s0022-5347(17)38803-1.
- Levitt MA, Mak GZ, Falcone RA, Peña A. Cloacal exstrophy–pull-through or permanent stoma? J Pediatr Surg 2008; 43 (1): 164–170. DOI: 10.1016/j.jpedsurg.2007.09.039.
- Hurwitz RS, Manzoni GAM, Ransley PG, Stephens FD. Cloacal Exstrophy: A Report of 34 Cases. J Urol 1987; 138 (4 Part 2): 1060–1064. DOI: 10.1016/s0022-5347(17)43502-6.
- Thauvin-Robinet C, Faivre L, Cusin V, Khau Van Kien P, Callier P, Parker KL, et al.. Cloacal exstrophy in an infant with 9q34.1-qter deletion resulting from a de novo unbalanced translocation between chromosome 9q and Yq. Am J Med Genet 2004; 126a (3): 303–307. DOI: 10.1002/ajmg.a.20596.
- Rickham PP. Vesicointestinal Fissure. Anorectal Malformations in Children 1960; 35 (97): 239–249. DOI: 10.1007/978-3-540-31751-7_14.
- Mathews R, Jeffs RD, Reiner WG, Docimo SG, Gearhart JP. Cloacal Exstrophy-improving The Quality Of Life. J Urol 1998; 160 (2452): 2452–2456. DOI: 10.1097/00005392-199812020-00017.
- M K. OEIS Complex (Omphalocele, Exstrophy of Bladder, Imperforate Anus and Spine Defects). Journal of Human Anatomy 2001; 2 (1). DOI: 10.23880/jhua-16000122.
- Appignani BA, Jaramillo D, Barnes PD, Poussaint TY. Dysraphic myelodysplasias associated with urogenital and anorectal anomalies: prevalence and types seen with MR imaging. AJR Am J Roentgenol 1994; 163 (5): 1199–1203. DOI: 10.2214/ajr.163.5.7976901.
- McLaughlin KP, Rink RC, Kalsbeck JE, Keating MA, Adams MC, King SJ, et al.. Cloacal Exstrophy: The Neurological Implications. J Urol 1995; 154 (2): 782–784. DOI: 10.1016/s0022-5347(01)67162-3.
- Karrer FM, Flannery AM, Nelson MD, McLone DG, Raffensperger JG. Anorectal malformations: Evaluation of associated spinal dysraphic syndromes. J Pediatr Surg 1988; 23 (1): 45–48. DOI: 10.1016/s0022-3468(88)80538-4.
- Dick EA, Bruyn R de, Patel K, Owens CM. Spinal Ultrasound in Cloacal Exstrophy. Clin Radiol 2001; 56 (4): 289–294. DOI: 10.1053/crad.2000.0648.
- Husmann DA, Vandersteen DR, Mclorie GA, Churchill BM. Urinary Continence After Staged Bladder Reconstruction For Cloacal Exstrophy: The Effect Of Coexisting Neurological Abnormalities On Urinary Continence. J Urol 1999; 161 (5): 1598–1602. DOI: 10.1016/s0022-5347(05)68990-2.
- Schlegel PN, Gearhart JP. Neuroanatomy of the Pelvis in an Infant with Cloacal Exstrophy: A Detailed Microdissection with Histology. J Urol 1989; 141 (3 Part 1): 583–585. DOI: 10.1016/s0022-5347(17)40901-3.
- Cohen AR. The Mermaid Malformation: Cloacal Exstrophy and Occult Spinal Dysraphism. Neurosurgery 1991; 28 (6): 834–843. DOI: 10.1227/00006123-199106000-00008.
- Sponseller PD, Bisson LJ, Gearhart JP, Jeffs RD, Magid D, Fishman E. The anatomy of the pelvis in the exstrophy complex. J Bone Joint Surg Am 1995; 77 (2): 177–189. DOI: 10.2106/00004623-199502000-00003.
- Stec AAJ, Wakim A, Barbet P, McCarthy EF, Lakshmanan Y, Sponseller PD, et al.. Fetal bony pelvis in the bladder exstrophy complex: normal potential for growth? Urology 2003; 62 (2): 337–341. DOI: 10.1016/s0090-4295(03)00474-6.
- Williams AM, Solaiyappan M, Pannu HK, Bluemke D, Shechter G, Gearhart JP. 3-dimensional Magnetic Resonance Imaging Modeling Of The Pelvic Floor Musculature In Classic Bladder Exstrophy Before Pelvic Osteotomy. J Urol 2004; 172 (4 Part 2): 1702–1705. DOI: 10.1097/01.ju.0000140212.56826.4c.
- Sugar EC, Firlit CF. Management of cloacal exstrophy. Urology 1990; 32 (4): 320–322. DOI: 10.1016/0090-4295(88)90234-8.
- Greene WB, Dias LS, Lindseth RE, Torch MA. Musculoskeletal problems in association with cloacal exstrophy. J Bone Joint Surg Am 1991; 73 (4): 551–560. DOI: 10.2106/00004623-199173040-00012.
- Baird AD, Nelson CP, Gearhart JP. Modern staged repair of bladder exstrophy. J Pediatr Urol 2007; 3 (311). DOI: 10.18591/bjuik.0243.
- Purves JT, Baird AD, Gearhart JP. The modern staged repair of bladder exstrophy in the female: A contemporary series. J Pediatr Urol 2008; 4 (2): 150–153. DOI: 10.1016/j.jpurol.2007.08.003.
- Hanna MK, Williams DI. Genital Function In Males With Vesical Exstrophy And Epispadias. Br J Urol 1969; 44 (2): 169–174. DOI: 10.1111/j.1464-410x.1972.tb10062.x.
- Stein R, Stöckle M, Fisch M, Nakai H, Müller SC, Hohenfellner R. The Fate Of The Adult Exstrophy Patient. J Urol 1994; 152 (5 Part 1): 1413–1416. DOI: 10.1016/s0022-5347(17)32433-3.
- Bastuba MD, Alper MM, Oates RD. Fertility and the use of assisted reproductive techniques in the adult male exstrophy/epispadias patient. Fertil Steril 1993; 60 (4): 733–736. DOI: 10.1016/s0015-0282(16)56234-7.
- Woodhouse CRJ. Sexual function in boys born with exstrophy, myelomeningocele, and micropenis. Urology 1998; 52 (1): 3–11. DOI: 10.1016/s0090-4295(98)00121-6.
- Ben-Chaim J, Jeffs RD, Reiner WG, Gearhart JP. The Outcome of Patients with Classic Bladder Exstrophy in Adult Life. The Exstrophy–Epispadias Complex 1996; 155 (1251): 169–173. DOI: 10.1007/978-1-4757-3056-2_26.
- Kropp BP, Cheng EY. Total Urogenital Complex Mobilization In Female Patients With Exstrophy. J Urol 2000; 164 (1035): 1035–1039. DOI: 10.1097/00005392-200009020-00028.
- Woodhouse CRJ. The gynaecology of exstrophy. BJU Int 1999; 83 (S3): 34–38. DOI: 10.1046/j.1464-410x.1999.0830s3034.x.
- Mathews RI, Gan M, Gearhart JP. Urogynaecological and obstetric issues in women with the exstrophy-epispadias complex. BJU Int 2003; 91 (9): 845–849. DOI: 10.1046/j.1464-410x.2003.04244.x.
- Canalichio KL, Ahn J, Artigas P, Amies Oelschlager A-ME, Rowe C, Merguerian P, et al.. Patient-reported outcomes in adult females with bladder exstrophy: A study of long-term sexual, reproductive and urinary outcomes using social media. J Pediatr Urol 2020; 16 (5): 567.e1–567.e7. DOI: 10.1016/j.jpurol.2020.06.020.
- Canalichio KL, Ahn J, Hwang C, Amies AM, Merguerian P, Shnorhavorian M. Faculty Opinions recommendation of Long-term urological and gynecological outcomes following complete primary repair in females with bladder exstrophy. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2021; 7 (5): 08 1–608 8. DOI: 10.3410/f.740664374.793590655.
- Giron AM, Passerotti CC, Nguyen H, Cruz JASda, Srougi M. Bladder exstrophy: reconstructed female patients achieving normal pregnancy and delivering normal babies. Int Braz J Urol 2011; 37 (5): 605–610. DOI: 10.1590/s1677-55382011000500006.
- Kramer SA, Jackson IT. Bilateral Rhomboid Flaps for Reconstruction of the External Genitalia in Epispadias-Exstrophy. Plast Reconstr Surg 1986; 77 (4): 621–629. DOI: 10.1097/00006534-198604000-00019.
- Krisiloff M, Puchner PJ, Tretter W, Macfarlane MT, Lattimer JK. Pregnancy in Women with Bladder Exstrophy. J Urol 1978; 119 (4): 478–479. DOI: 10.1016/s0022-5347(17)57522-9.
- Burbige KA, Hensle TW, Chambers WJ, Leb R, Jeter KF. Pregnancy and sexual function in women with bladder exstrophy. Urology 1986; 28 (1): 12–14. DOI: 10.1016/0090-4295(86)90172-x.
- Kennedy WA, Hensle TW, Reiley EA, Fox HE, Haus T. Pregnancy after orthotopic continent urinary diversion. Int J Gynaecol Obstet 1993; 46 (1): 88–89. DOI: 10.1016/0020-7292(94)90337-9.
- Stein R, Fisch M, Bauer H, Friedberg V, Hohenfellner R. Operative Reconstruction of the External and Internal Genitalia in Female Patients with Bladder Exstrophy or Incontinent Epispadias. J Urol 1995; 154 (1002): 1002–1007. DOI: 10.1097/00005392-199509000-00026.
- Mednick L, Gargollo P, Oliva M, Grant R, Borer J. Stress and Coping of Parents of Young Children Diagnosed With Bladder Exstrophy. J Urol 2009; 181 (3): 1312–1317. DOI: 10.1016/j.juro.2008.10.051.
- Meldrum KK, Mathews RI, Nelson CP, Gearhart JP. Subspecialty training and surgical outcomes in children with failed bladder exstrophy closure. J Pediatr Urol 2005; 1 (2): 95–99. DOI: 10.1016/j.jpurol.2005.01.002.
- Nelson CP, North AC, Ward MK, Gearhart JP. Economic Impact of Failed or Delayed Primary Repair of Bladder Exstrophy: Differences in Cost of Hospitalization. J Urol 2008; 179 (2): 680–683. DOI: 10.1016/j.juro.2007.09.093.
- Nelson CP, Dunn RL, Wei JT, Gearhart JP. Surgical Repair Of Bladder Exstrophy In The Modern Era: Contemporary Practice Patterns And The Role Of Hospital Case Volume. J Urol 2005; 174 (3): 1099–1102. DOI: 10.1097/01.ju.0000169132.14799.33.
- Bethell G, Johal N, Cuckow P. Cloacal Exstrophy Repair with Primary Closure of Bladder Exstrophy: A Case Report and Review of Literature. Case Rep Pediatr 1952; 2016 (654): 1–3. DOI: 10.1155/2016/8538935.
- Cendron l., Zanotti G, Percudani R, Ramazzina I, Puggioni V, Maccacaro E, et al.. Crystal structure of allantoin racemase from Pseudomonas fluorescens AllR. Ann Chir Infant 1971; 12 (371). DOI: 10.2210/pdb5lfd/pdb.
- Jeffs RD, Charrios R, Mnay M. Primary closure of the exstrophied bladder. In: Scott R, editor. Current Controversies in Urologic Management. Philadelphia:WB Saunders; 1972. DOI: 10.1097/00006534-195701000-00025.
- Landes RR, Melnick I, Klein R. Vesical exstrophy with epispadias twenty-year follo-up. Urology 1973; 9 (1): 53–56. DOI: 10.1016/0090-4295(77)90285-0.
- Gearhart JP, Jeffs RD. State-of-the-Art Reconstructive Surgery for Bladder Exstrophy at The Johns Hopkins Hospital. Arch Pediatr Adolesc Med 1989; 143 (12): 1475. DOI: 10.1001/archpedi.1989.02150240097026.
- Grady RW, Mitchell ME. Complete Primary Repair Of Exstrophy. J Urol 1999; 162 (4): 1415–1420. DOI: 10.1016/s0022-5347(05)68327-9.
- Chan DY, Jeffs RD, Gearhart JP. Determinants of continence in the bladder exstrophy population: predictors of success? Urology 2001; 57 (4): 774–777. DOI: 10.1016/s0090-4295(00)01102-x.
- Stein R, Fisch M, Black P, Hohenfellner R. Strategies For Reconstruction After Unsuccessful Or Unsatisfactory Primary Treatment Of Patients With Bladder Exstrophy Or Incontinent Epispadias. J Urol 1999; 161 (1934): 1934–1941. DOI: 10.1097/00005392-199906000-00066.
- Grady RW, Mitchell ME. Surgical techniques for one-stage reconstruction of the exstrophy-epispadias complex. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. Philadelphia: Saunders; 2007.
- Husmann DA. Surgery Insight: advantages and pitfalls of surgical techniques for the correction of bladder exstrophy. Nat Clin Pract Urol 2006; 3 (2): 95–100. DOI: 10.1038/ncpuro0407.
- Kibar Y, Roth CC, Frimberger D, Kropp BP. Our initial experience with the technique of complete primary repair for bladder exstrophy. J Pediatr Urol 2009; 5 (3): 186–189. DOI: 10.1016/j.jpurol.2008.11.005.
- Mitchell ME. Bladder exstrophy repair: Complete primary repair of exstrophy. Urology 2005; 65 (1): 5–8. DOI: 10.1016/j.urology.2004.07.030.
- Gargollo PC, Borer JG, Diamond DA, Hendren WH, Rosoklija I, Grant R, et al.. Prospective Followup in Patients After Complete Primary Repair of Bladder Exstrophy. J Urol 2008; 180 (4s): 1665–1670. DOI: 10.1016/j.juro.2008.05.076.
- Shnorhavorian M, Grady RW, Anderson A. Long-Term Followup of Complete Primary Repair of Exstrophy: The Seattle Experience. Yearbook of Urology 2008; 2009 (1615): 214–215. DOI: 10.1016/s0084-4071(09)79240-1.
- Hafez AT, El-Sherbiny MT. Complete Repair Of Bladder Exstrophy: Management Of Resultant Hypospadias. J Urol 2005; 173 (3): 958–961. DOI: 10.1097/01.ju.0000147012.49955.6f.
- Gearhart JP, Baird AD. The Failed Complete Repair Of Bladder Exstrophy: Insights And Outcomes. J Urol 2005; 174 (4 Part 2): 1669–1673. DOI: 10.1097/01.ju.0000175994.35468.2f.
- Gargollo PC, Borer JG. Contemporary outcomes in bladder exstrophy. Curr Opin Urol 2007; 17 (4): 272–280. DOI: 10.1097/mou.0b013e3281ddb32f.
- Shapiro E, Jeffs RD, Gearhart JP, Lepor H. Muscarinic Cholinergic Receptors in Bladder Exstrophy: Insights Into Surgical Management. J Urol 1985; 134 (2): 308–310. DOI: 10.1016/s0022-5347(17)47139-4.
- Lee BR, Perlman EJ, Partin AW, Jeffs RD, Gearhart JP. Evaluation of Smooth Muscle and Collagen Subtypes in Normal Newborns and Those With Bladder Exstrophy. J Urol 1996; 156 (203): 2034–2036. DOI: 10.1097/00005392-199612000-00042.
- Peppas DS, Tchetgen M-B, Lee BR, Jeffs RD, Gearhart JP. A Quantitative Histological Analysis of the Bladder in Classical Bladder Exstrophy in Various Stages of Reconstruction Utilizing Color Morphometry. The Exstrophy–Epispadias Complex 1999; 1999 (41): 41–47. DOI: 10.1007/978-1-4757-3056-2_7.
- Lais A, Paolocci N, Ferro F, Bosman C, Boldrini R, Caione P. Morphometric Analysis of Smooth Muscle in the Exstrophy-Epispadias Complex. J Urol 1996; 156 (819): 819–821. DOI: 10.1097/00005392-199608001-00074.
- Orsola A, Estrada CR, Nguyen HT, Retik AB, Freeman MR, Peters CA, et al.. Growth and stretch response of human exstrophy bladder smooth muscle cells: molecular evidence of normal intrinsic function. BJU Int 2005; 95 (1): 144–148. DOI: 10.1111/j.1464-410x.2004.05267.x.
- Mathews R, Wills M, Perlman E, Gearhart JP. Neural Innervation Of The Newborn Exstrophic Bladder. J Urol 1999; 162 (506): 506–508. DOI: 10.1097/00005392-199908000-00076.
- Rösch W, Christl A, Strauss B, Schrott K-M, Neuhuber WL. Comparison of Preoperative Innervation Pattern and Postreconstructive Urodynamics in the Exstrophy-Epispadias Complex. Urol Int 1997; 59 (1): 6–15. DOI: 10.1159/000283009.
- Hipp J, Andersson K-E, Kwon TG, Kwak EK, Yoo J, Atala A. Microarray analysis of exstrophic human bladder smooth muscle. BJU Int 2008; 0 (0): 070916224627009–??? DOI: 10.1111/j.1464-410x.2007.07211.x.
- Novak TE, Lakshmanan Y, Frimberger D, Epstein JI, Gearhart JP. Polyps In The Exstrophic Bladder. A Cause For Concern? J Urol 2005; 174 (4 Part 2): 1522–1526. DOI: 10.1097/01.ju.0000179240.25781.1b.
- Toguri AG, Churchill BM, Schillinger JF, Jeffs RD. Continence in Cases of Bladder Exstrophy. J Urol 1987; 119 (4): 538–540. DOI: 10.1016/s0022-5347(17)57541-2.
- Diamond DA, Bauer SB, Dinlenc C, Hendren WH, Peters CA, Atala A, et al.. Normal Urodynamics In Patients With Bladder Exstrophy: Are They Achievable? J Urol 1999; 162 (3 Part 1): 841–845. DOI: 10.1097/00005392-199909010-00072.
- Mathews R, Gosling JA, Gearhart JP. Ultrastructure Of The Bladder In Classic Exstrophy: Correlation With Development Of Continence. J Urol 2004; 172 (4 Part 1): 1446–1449. DOI: 10.1097/01.ju.0000138248.43831.27.
- Wakim A, Barbet JP. Connections of the bladder plate and bladder neck with the bony pelvis in a fetus with classic bladder exstrophy. Urology 2002; 60 (1): 142–146. DOI: 10.1016/s0090-4295(02)01715-6.
- Gearhart JP, Mathews R. The Failed Exstrophy Closure. The Exstrophy–Epispadias Complex 1991; 18 (687): 93–96. DOI: 10.1007/978-1-4757-3056-2_15.
- Connolly JA, Peppas DS, Jeffs RD, Gearhart JP. Prevalence and Repair of Inguinal Hernias in Children with Bladder Exstrophy. J Urol 1995; 154 (1900): 1900–1901. DOI: 10.1097/00005392-199511000-00093.
- Baker LA, Gearhart JP. The staged approach to bladder exstrophy closure and the role of osteotomies. World J Urol 1998; 16 (3): 205–211. DOI: 10.1007/s003450050054.
- Silver RI, Yang A, Ben-Chaim J, Jeffs RD, Gearhart JP. Penile Length in Adulthood after Exstrophy Reconstruction. The Exstrophy–Epispadias Complex 1997; 158 (999): 117–125. DOI: 10.1007/978-1-4757-3056-2_19.
- Gearhart JP, Yang A, Leonard MP, Jeffs RD, Zerhouni EA. Prostate Size and Configuration in Adults with Bladder Exstrophy. J Urol 1993; 149 (2): 308–310. DOI: 10.1016/s0022-5347(17)36064-0.
- Berkowitz J, Carter HB, Gearhart JP. Prostate Cancer in Patients with the Bladder Exstrophy-Epispadias Complex: Insights and Outcomes. Urology 2008; 71 (6): 1064–1066. DOI: 10.1016/j.urology.2007.12.069.
- D’Hauwers KWM, Feitz WFJ, Kremer JAM. Bladder exstrophy and male fertility: pregnancies after ICSI with ejaculated or epididymal sperm. Fertil Steril 2008; 89 (2): 387–389. DOI: 10.1016/j.fertnstert.2007.03.005.
- Cadeddu JA, Benson JE, Silver RI, Lakshmanan Y, Jeffs RD, Gearhart JP. Spinal abnormalities in classic bladder exstrophy. BJU Int 1997; 79 (6): 975–978. DOI: 10.1046/j.1464-410x.1997.00190.x.
- Halachmi S, Farhat W, Konen O, Khan A, Hodapp J, Bagli DJ, et al.. Pelvic Floor Magnetic Resonance Imaging after Neonatal Single Stage Reconstruction in Male Patients With Classic Bladder Exstrophy. J Urol 2003; 170 (4 Part 2): 1505–1509. DOI: 10.1097/01.ju.0000087463.92231.b1.
- Gargollo PC, Borer JG, Retik AB, Peters CA, Diamond DA, Atala A, et al.. Magnetic Resonance Imaging Of Pelvic Musculoskeletal And Genitourinary Anatomy In Patients Before And After Complete Primary Repair Of Bladder Exstrophy. J Urol 2005; 174 (4 Part 2): 1559–1566. DOI: 10.1097/01.ju.0000175997.60933.fe.
- Ebert AK, Falkert A, Brandl R, Hirschfelder H, Koller M, RÃ\textparagraphsch WH. Pelvic-floor imaging using three-dimensional ultrasonography and magnetic resonance imaging in the long term follow-up of the bladder-exstrophy-epispadias complex. BJU Int 2010; 105 (2): 248–253. DOI: 10.1111/j.1464-410x.2009.08736.x.
- Ives E, Coffey R, Carter CO. A family study of bladder exstrophy. J Med Genet 1980; 17 (2): 139–141. DOI: 10.1136/jmg.17.2.139.
- Reutter H, Qi L, Gearhart JP, Boemers T, Ebert A-K, Rösch W, et al.. Concordance analyses of twins with bladder exstrophy–epispadias complex suggest genetic etiology. Am J Med Genet A 2007; 143a (22): 2751–2756. DOI: 10.1002/ajmg.a.31975.
- Boyadjiev SA, Dodson JL, Radford CL, Ashrafi GH, Beaty TH, Mathews RI, et al.. Clinical and molecular characterization of the bladder exstrophy-epispadias complex: analysis of 232 families. BJU Int 2004; 94 (9): 1337–1343. DOI: 10.1111/j.1464-410x.2004.05170.x.
- Wood HM, Trock BJ, Gearhart JP. In Vitro Fertilization and the Cloacal-Bladder Exstrophy-Epispadias Complex: Is there an Association? J Urol 2003; 169 (4): 1512–1515. DOI: 10.1097/01.ju.0000054984.76384.66.
- Wood HM, Babineau D, Gearhart JP. In vitro fertilization and the cloacal/bladder exstrophy–epispadias complex: A continuing association. J Pediatr Urol 2007; 3 (4): 305–310. DOI: 10.1016/j.jpurol.2006.10.007.
- Boyadjiev SA, South ST, Radford CL, Patel A, Zhang G, Hur DJ, et al.. A reciprocal translocation 46,XY,t(8;9)(p11.2;q13) in a bladder exstrophy patient disrupts CNTNAP3 and presents evidence of a pericentromeric duplication on chromosome 9. Genomics 2004; 85 (5): 622–629. DOI: 10.1016/j.ygeno.2005.01.002.
- Reutter H, Thauvin-Robinet C, Boemers TM, Rösch WH, Ludwig M. Bladder exstrophy–epispadias complex: Investigation of suppressor of variegation, enhancer of zeste and Trithorax (SET) as a candidate gene in a large cohort of patients. Scand J Urol Nephrol 2006; 40 (3): 221–224. DOI: 10.1080/00365590600621204.
- Ludwig M, Ruschenforf F, Saar K, Hubner N, Siekmann L, Boyadjiev SA, et al.. Genome-wide linkage scan for bladder exstrophy-epispadias complex. Birth Defects Res A Clin Mol Teratol. 2009; 5 (2): 74–78.
- Gearhart J, Benchaim J, Jeffs R, Sanders R. Criteria for the prenatal diagnosis of classic bladder exstrophy. Obstet Gynecol 1995; 85 (6): 961–964. DOI: 10.1016/0029-7844(95)00069-4.
- Gobbi D, Fascetti Leon F, Tregnaghi A, Gamba PG, Midrio P. Early Prenatal Diagnosis of Cloacal Exstrophy with Fetal Magnetic Resonance Imaging. Fetal Diagn Ther 2008; 24 (4): 437–439. DOI: 10.1159/000174570.
- Lattimer JK, Vernon Smith MJ. Exstrophy Closure: a Followup on 70 Cases. J Urol 1966; 95 (3): 356–359. DOI: 10.1016/s0022-5347(17)63460-8.
- Birth Defects Monitoring Systems IC for. Epidemiology of bladder exstrophy and epispadias: A communication from the international clearinghouse for birth defects monitoring systems. Teratology 1987; 36 (2): 221–227. DOI: 10.1002/tera.1420360210.
- Nelson CP, Dunn RL, Wei JT. Contemporary Epidemiology Of Bladder Exstrophy In The United States. J Urol 2005; 173 (5): 1728–1731. DOI: 10.1097/01.ju.0000154821.21521.9b.
- Ambrose SS, O’Brien DP. Surgical Embryology of the Exstrophy-Epispadias Complex. Surg Clin North Am 1974; 54 (6): 1379–1390. DOI: 10.1016/s0039-6109(16)40493-7.
- Marshall VF, Muecke C. Congenital abnormalities of the bladder. Handbuch der Urologie. New York: Springer Verlag; 1968. DOI: 10.1007/978-3-642-87399-7_4.
- Mildenberger H, Kluth D, Dziuba M. Embryology of bladder exstrophy. J Pediatr Surg 1988; 23 (2): 166–170. DOI: 10.1016/s0022-3468(88)80150-7.
- Johnson JH, Kogan SJ. The exstrophic anomalies and their surgical reconstruction. Curr Prob Surg 1974 (August):1-3. DOI: 10.1016/s0011-3840(74)80011-0.
- Rubenwolf P, Thomas C, Thüroff JW, Stein R. Sexual Function and Fertility of Women with Classic Bladder Exstrophy and Continent Urinary Diversion. J Urol 2016; 196 (1): 140–145. DOI: 10.1016/j.juro.2015.12.099.
Last updated: 2025-09-25 12:10