16: Vesicoureteral Reflux
阅读本章大约需要 35 分钟。
Introduction
Vesicoureteral reflux (VUR) is the retrograde flow of urine from the bladder into the ureters and sometimes kidneys. VUR is a risk factor for recurrent pyelonephritis, renal scarring, renal insufficiency and hypertension and is cited as a cause of end-stage renal disease in children.1,2,3,4 Management of reflux is primarily aimed at reducing the development of these long-term adverse effects through prevention of pyelonephritis.
A paradigm shift in the evaluation and management of VUR has occurred over the last several decades, and clinicians should be aware of controversies in VUR management. It appears that active treatment of reflux has improved outcomes for some children such that the incidence of reflux-associated nephropathy continues to decline, but recent urologic literature suggests that the natural history of VUR does not follow a uniform pathway. Many children with reflux do not benefit from either diagnosis or treatment, as there is a high rate of spontaneous resolution without adverse effects.5,6 Clarification of which children do benefit from treatment is the greatest challenge to the advancement of vesicoureteral reflux management.
Background, Incidence, and Pathophysiology
VUR occurs in approximately 1-3% of children, and 2.3% of new-onset ESRD patients aged 0-21 in the United States are reported to have a primary diagnosis attributed to reflux nephropathy. Incidence of ESRD with reflux nephropathy as a primary etiology has decreased steadily over the last twenty years.7 Previous international studies have estimated a higher prevalence of about 10%.5,8
Although anatomic references to the concept of reflux were made as early as the first century AD, pivotal studies in the 1950s first contributed to our understanding of the association of VUR, chronic pyelonephritis and renal scarring as we understand it today.9,10
VUR can be categorized as either primary or secondary. Primary VUR is attributed to an abnormally short intramural portion of ureter tunnelling through the detrusor muscle at the ureterovesical junction. In a normal junction, the intramural ureter is passively compressed during bladder filling, creating an antireflux mechanism. This mechanism is dependent on intramural length-to-diameter ratio and ureterovesical insertion angle.11
A short intravesical tunnel is associated with VUR, and the tunnel length is inversely proportional to the severity of the reflux.12
Secondary VUR develops when abnormal lower urinary tract function and elevated intravesical pressures overcome the antireflux mechanism and is associated with conditions like bladder outlet obstruction (e.g., posterior urethral valve) or neurogenic bladder. Secondary VUR may also be seen in children with no anatomic genitourinary or neurologic abnormality, but suffering from bladder and bowel dysfunction.13 The most frequent cause of secondary reflux is non-neurogenic bowel and bladder dysfunction, which is also one of the greatest risk factors for breakthrough UTIs.
Inheritance
A strong inheritance pattern exists with primary VUR, with up to 80% of identical and 35% of fraternal twins concordant for presence of VUR.2 Multiple polymorphisms associated with abnormalities in ureteral budding have been identified in patients with primary VUR, although no single gene has been found to predominate.14 The probability of a non-twin sibling having reflux is about 25% and the chances of offspring having reflux are approximately 35-50%.15,16 Interestingly, this relationship is not as strong in children with dysfunctional elimination which likely speaks to the secondary nature of VUR in this cohort.17
Vesicoureteral Reflux, Urinary Tract Infections, and Renal Scarring
The clinical significance of VUR lies largely in its association with congenital renal dysplasia as well as renal parenchymal damage occurring secondary to recurrent pyelonephritis. At least one-third of VUR patients have renal scars.18,19 The presence of scarring implies regions of renal damage and increases the risk for long-term adverse sequelae.
Congenital Reflux Nephropathy
In newborns with VUR, scars have been detected in association with high-grade reflux before the occurrence of clinical UTI.20 These “congenital scars” are thought to be regions of focal dysplasia or hypoplasia resulting from abnormal nephrogenesis as opposed to damaged normal tissue following pyelonephritis. Congenital renal dysplasia may be indistinguishable from acquired renal scars secondary to infection. Since DMSA scans are not routinely performed on neonates, it is unknown what proportion of renal scars attributed to infectious injury are actually due to abnormalities of embryogenesis.21 While the ultimate etiology remains uncertain, they are regions of diminished renal function and can be associated with significant morbidity and mortality independent of the development of infection-associated renal scarring.22
Pyelonephritis and Renal Scarring
Urinary tract infections are common in children, affecting about 5% of girls and about half as many boys.23 An estimated 30-40% of children under the age of 5 who develop urinary tract infections have VUR upon further evaluation.24,25 Renal scarring is believed to occur when infected urine comes in contact with renal parenchyma causing an inflammatory reaction; it has been associated with an increased risk of hypertension, proteinuria, and renal insufficiency.3,26
VUR as a factor predisposing to renal injury due to infection was first recognized in spinal cord injury patients. After surgical correction, these children had fewer episodes of pyelonephritis and urosepsis.27 Further studies demonstrated a lower rate of new renal scars developing in children with primary reflux compared to those with secondary reflux from neurogenic bladder or voiding dysfunction.28,29
Permanent renal injury resulting from pyelonephritis can be identified as renal scarring on 99mTc dimercaptosuccinic acid (DMSA) scanning. In a meta-analysis examining the presence of renal damage in children hospitalized with urinary tract infection, approximately 34% of children with pyelonephritis had VUR, and of those with VUR and pyelonephritis, 72% had an abnormal DMSA scan.30
Renal scarring on nuclear scintigraphy may be detectable during or shortly after an acute episode of pyelonephritis. The sequelae from renal scars may not become apparent for many years; as long as 30-40 years have been reported between first renal-scarring pyelonephritis and the development of hypertension or end-stage renal disease.27 The prolonged time between the apparent initial renal insult and the clinically apparent effects underscores the need for long-term follow-up of patients with VUR.
Reflux Grading and Other Predictors of Outcomes
Because there is a high rate of spontaneous resolution of VUR in most children but potentially devastating clinical sequelae in others, identifying those most at risk is a primary aim of VUR management. The International Reflux Study classified VUR along a 5-point scale defined by the degree of retrograde urine flow and the accompanying distortion of the pyelocalyceal system (Figure 1)31 Higher grades are associated with decreased spontaneous resolution rates and increased prevalence of renal scars.23,32
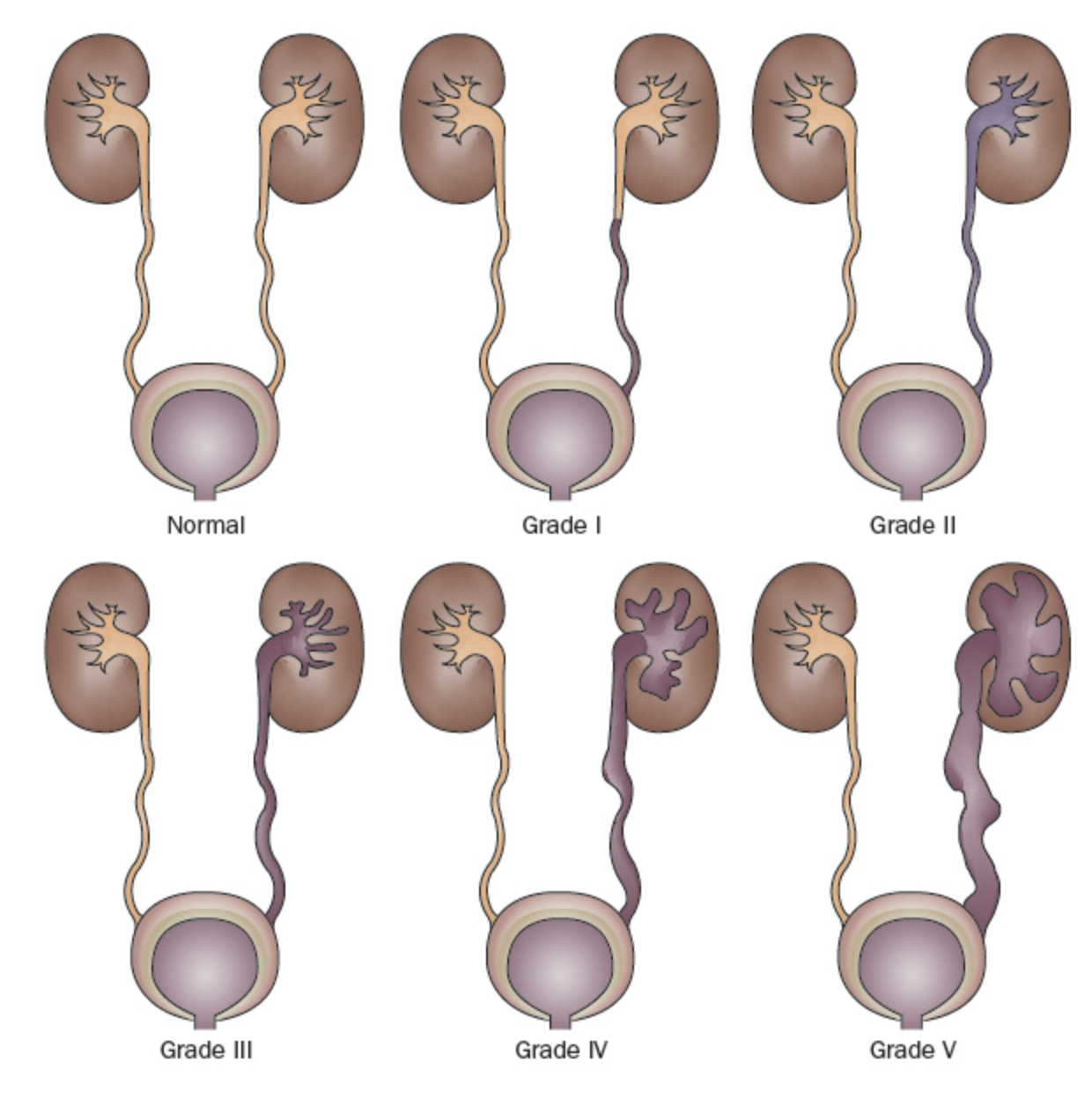
Figure 1 International Reflux Grading System.31
- Grade I: reflux into the ureter only
- Grade II: reflux into a non-dilated pyelocalyceal system
- Grade III: dilatation of the collecting system (Figure 2)
- Grade IV: more extensive dilation with blunting of the calyces and tortuosity of the ureter (Figure 3)
- Grade V: massive dilation of the collecting system and severe tortuosity of the ureter (Figure 4)
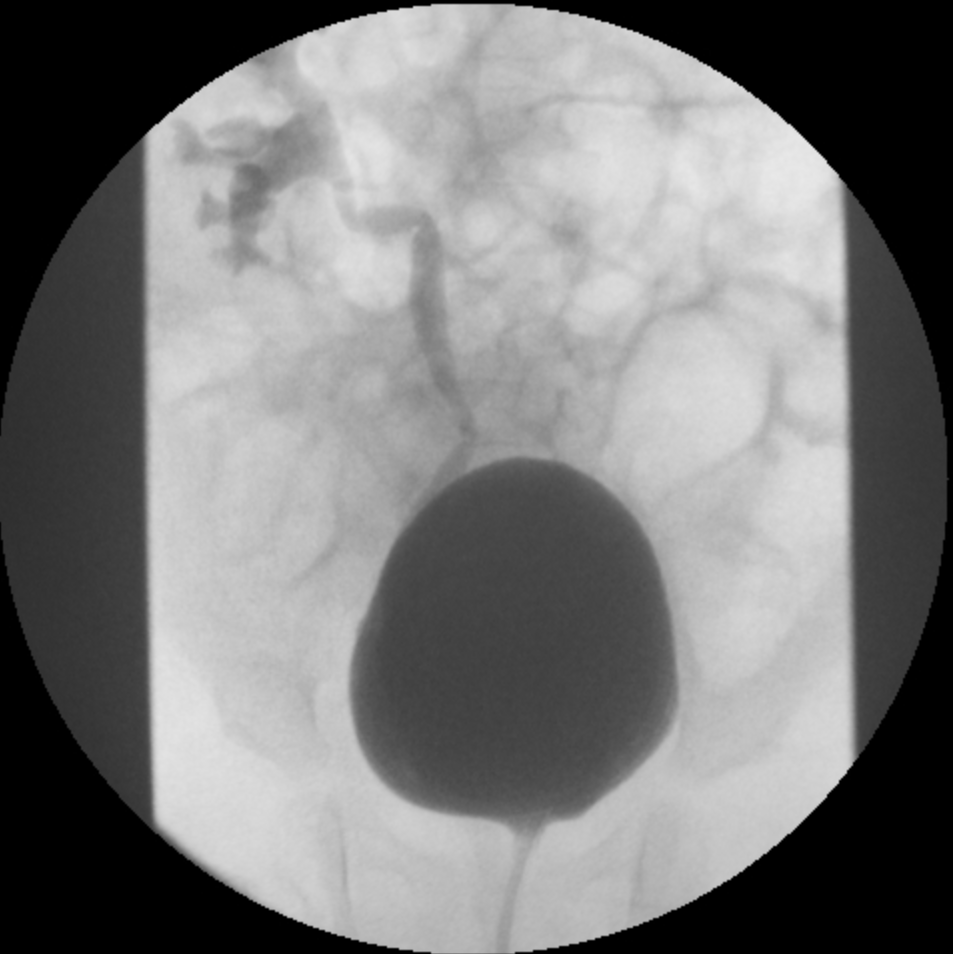
Figure 2 Grade III VUR
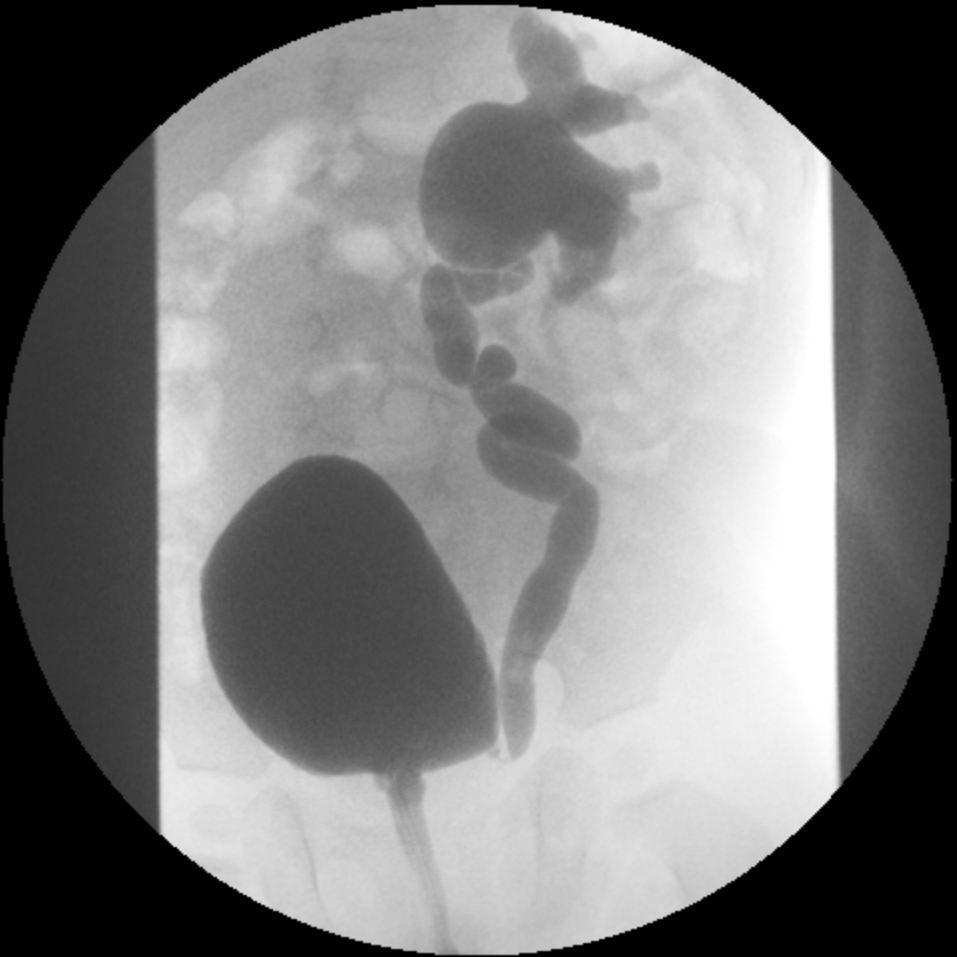
Figure 3 Grade IV VUR
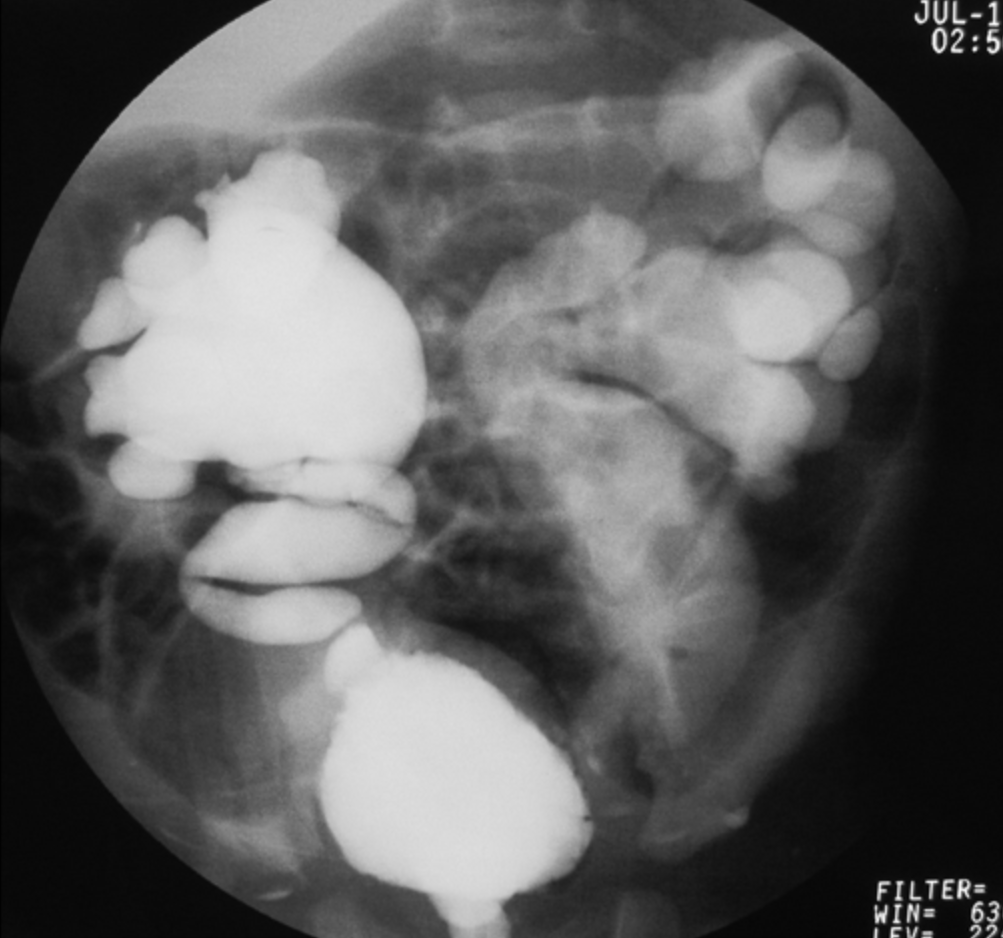
Figure 4 Grade V VUR
Multiple studies demonstrate a direct correlation between prevalence of renal scarring and grade of reflux..33 Renal scarring develops less often in non-dilating reflux.22,34,35 The chance of developing further renal parenchymal loss has been shown to be higher in children with grades III-V reflux than in those with grades I-II.36,37 Renal scars have also been demonstrated to be a negative predictor of VUR resolution independent of reflux grade.38 Unfortunately, the international reflux grading system is subjective with poor inter-rater reliability.39,40
More recently, additional factors influencing reflux resolution and breakthrough UTIs have been identified. Clinical factors predictive of reflux resolution, risk of renal injury and/or breakthrough UTIs include age, gender (female gender increases risk), circumcision status (circumcision is protective for UTI), presence of bladder and bowel dysfunction, and prior history of UTI. Radiographic predictors include laterality (unilateral vs. bilateral), distal ureteral diameter ratio (relative to the L1-L3 intervertebral body distance),41,42,43,44,45 bladder volume at the onset of reflux (normalized as a percentage of age predicted bladder capacity),46 bladder pressure at the onset of reflux, whether reflux occurs in the filling or voiding phase, and presence of renal scars.38,47
For neonates, a six-point vesicoureteral reflux index utilizing gender, VUR grade, anatomic ureteral abnormalities, and time of reflux during VCUG was shown to be predictive of VUR resolution, time to improvement, and breakthrough UTI.48,49 Similarly, a user-friendly neural network has been created utilizing demographic information, VUR grade, laterality, bladder volume at onset of VUR, UTI history, bladder or bowel dysfunction, and presence of breakthrough febrile UTIs to predict likelihood of VUR resolution, and is available at http://pedsurocomp.lab.uiowa.edu.50 Consideration of multiple risk factors improves prediction of clinical outcomes and permits more tailored individualized management of each patient.
Bowel and Bladder Dysfunction
Children with bowel or bladder dysfunction have been consistently shown to have higher incidence of breakthrough UTIs, more renal scarring, a lower spontaneous resolution rate of VUR, and a higher failure rate following surgical treatment than children with “normal” elimination habits.1,51 These findings should be taken into consideration when determining management options for potty-trained children with VUR.
Reflux Nephropathy/Renal Scarring
Children with renal scars are more likely to develop further UTIs and additional scars than children without renal scars.34,52,53 One retrospective study of 120 patients demonstrated a significantly higher chance of developing a breakthrough urinary tract infection in those with grades III-V reflux and an abnormality on baseline DMSA scan (60%) compared to those without an abnormality (6%), and another study showed a relative risk of breakthrough UTI of 5.1 in patients with renal scarring.54,55 In a study with mean follow-up of 12 years after an anti-reflux operation, children with unilateral renal scars had an 11% chance of developing hypertension and an 18.5% chance if they had bilateral renal scars.56 Others have suggested the incidence of hypertension in children with bilateral renal scars is about 20%.57 Children with severe bilateral renal scars are significantly more likely to develop proteinuria, chronic renal insufficiency and renal failure than those with unilateral scars or unscarred kidneys.58,59 These data strongly suggest that children with scarring are at increased risk for further development of additional scars and long-term clinical sequelae.
Diagnosis and Evaluation
Voiding Cystourethrography and Nuclear Cystogram
The only tests that routinely and reliably detect reflux are voiding cystourethrography (VCUG) and nuclear cystography. A VCUG is performed by instilling contrast retrograde via a urethral catheter into the bladder and obtaining fluoroscopic images of the upper and lower urinary tracts during filling and voiding. A nuclear cystogram is performed using a radioisotope scanning technique. VCUG is an appropriate initial test as it provides better anatomic details, including the presence or absence of periureteral diverticula, ureteral duplication, and bladder or urethral abnormalities. It also allows for more precise grading of reflux. In addition, it permits measurement of the distal ureteral diameter which has been shown to be more objective and reliable than grade and of equal or greater predictive value in terms of resolution and clinical outcomes.41,42,43,44,45
Nuclear cystography is beneficial in that it reduces radiation dose compared to VCUG. Nuclear cystography reliably detects all grades of vesicoureteral reflux and may be more sensitive for the detection of intermittent VUR (Figure 5)60,61
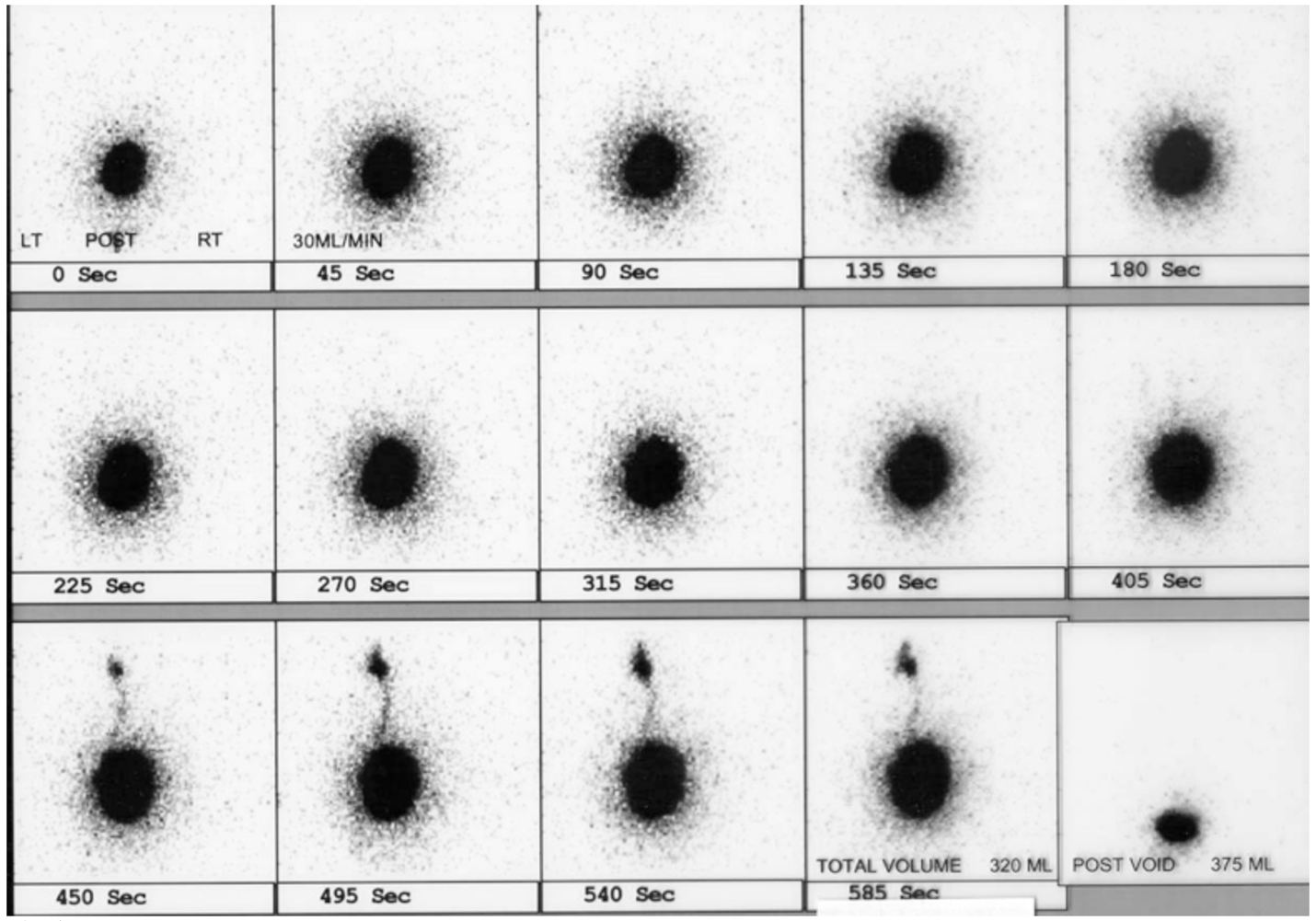
Figure 5 Left VUR on nuclear cystogram
If a VCUG demonstrates no significant anatomic abnormalities, nuclear cystography may be performed for follow-up studies in order to limit radiation exposure. Although the nuclear cystogram is frequently considered a more sensitive test, the ordering practitioner should be aware that each type of cystogram has limitations to its ability to detect reflux in a given population.62
The American Academy of Pediatrics Sections on Urology and Radiology created a standard protocol for performance of VCUG in 2016 to maximize patient safety and ensure accurate results that directly affect patient management.63 Key recommendations included observing ALARA principles,64 filling the bladder by gravity at 100 cm above the exam table, obtaining images of the urethra during the voiding phase, performing a cyclic study (multiple fill cycles), estimating maximum bladder capacity, recording bladder volume at which time VUR occurs, recording postvoid residual, recording bladder or urethra abnormalities (e.g., bladder diverticuli or posterior urethral valve), and grading reflux according to the International Reflux Study.
In addition to radiation exposure, catheterization can be a traumatic experience for a young child. Efforts to decrease the traumatic nature of VCUG include the use of lubricants containing local anesthetics, use of child life specialists, and conscious sedation. Historically, cystograms were repeated annually; however, with improved ability to predict timing of resolution it has been suggested that in children less likely to resolve VUR, the interval between cystograms should be lengthened to decrease exposure to radiation, the number of traumatic studies, and cost.50,65,66,67
A relatively recently-adopted imaging modality in some centers is that of contrast-enhanced voiding urosonography (ceVUS), which has been shown in several studies to be safe and comparable to VCUG in detecting grade 2 or higher VUR, detecting intrarenal reflux and having good interobserver reliability (Figure 6) It allows complete avoidance of ionizing radiation but does still necessitate catheterization and intravesical instillation of the contrast agent. Second-generation ultrasound contrast agents were not widely available until recently FDA-approved in the United States.68,69 VCUG does remain the gold standard for diagnosis of VUR until further research can show equivalent sensitivity and specificity of other testing methods.
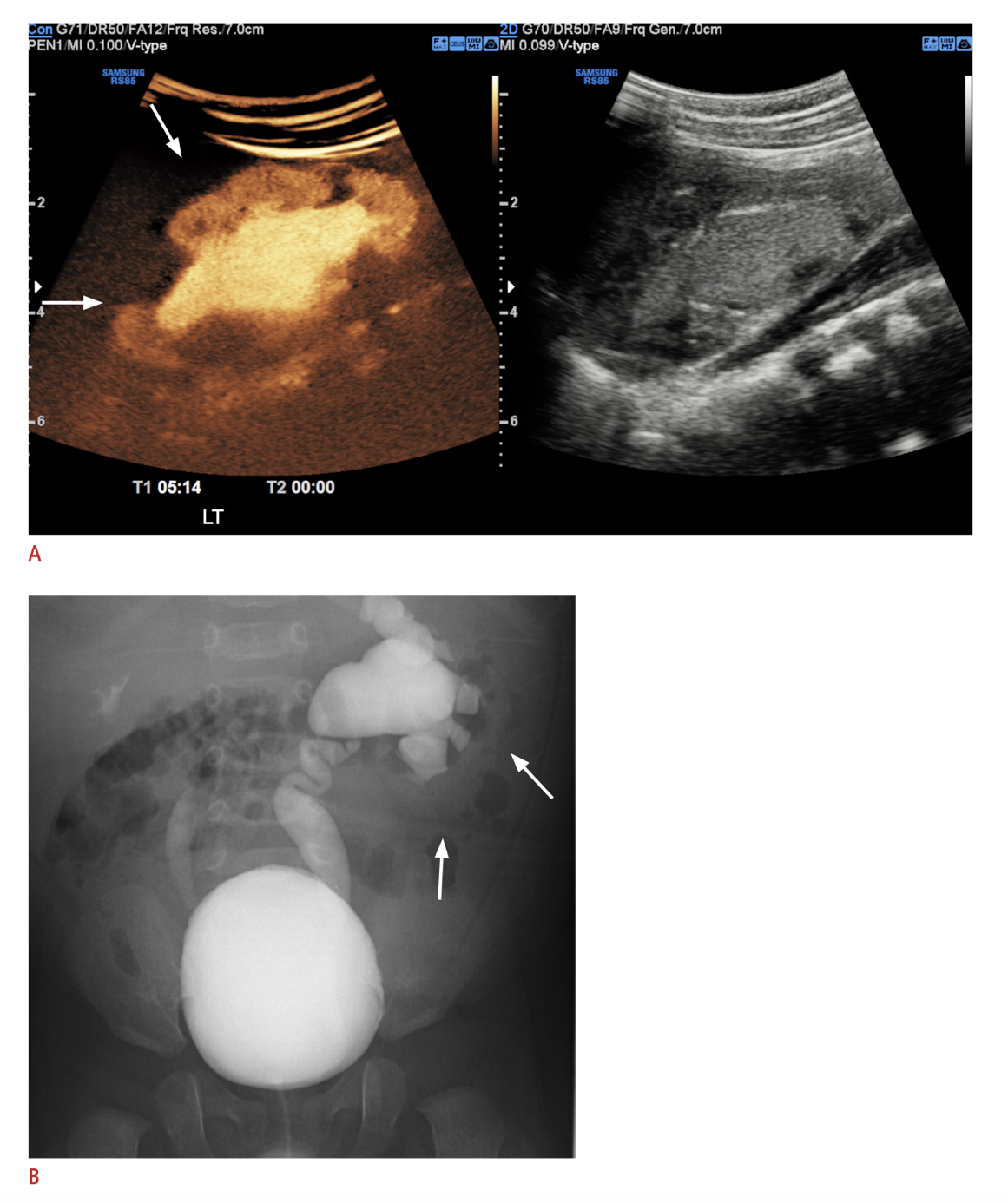
Figure 6 A. Contrast-enhanced ultrasound image of left kidney with intrarenal reflux compared with B. VCUG showing left grade V VUR and right grade III VUR. Image reproduced under terms of Creative Commons Attribution Non-Commercial License. Image reprinted from. Copyright 2021 Korean Society of Ultrasound in Medicine.
Nuclear Scintigraphy (DMSA, MAG3)
DMSA scintigraphy, in which the radioactive agent binds to the proximal tubules, has been found to be a more sensitive study than intravenous pyelogram (IVP) for the detection of reflux nephropathy.70 The scan provides information about differential renal function and can also detect changes of acute pyelonephritis with greater sensitivity and specificity than CT scan, magnetic resonance imaging, or ultrasound.71(Figure 7)
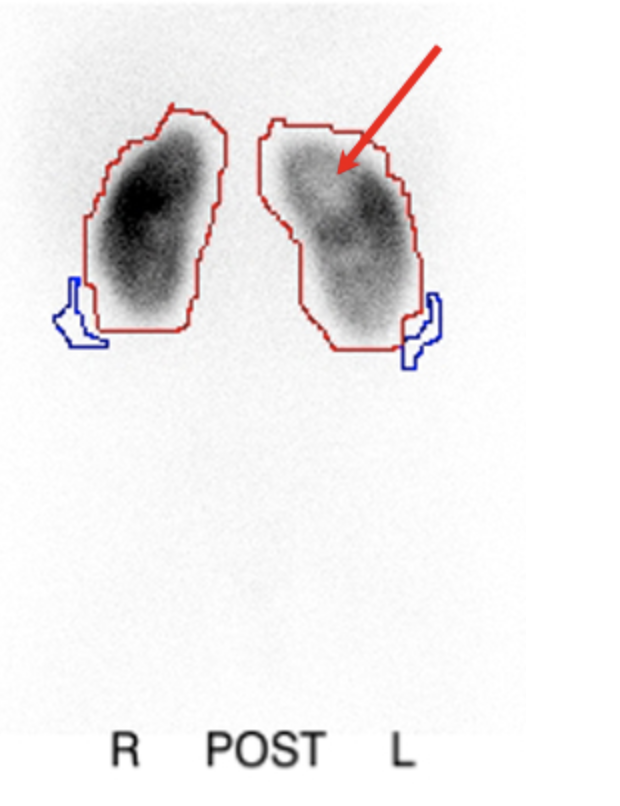
Figure 7 DMSA scintigraphy following pyelonephritis showing photopenic defects in left kidney
The association of renal scars with higher grades of reflux and risk for subsequent scars as well as decreased resolution rates lead some to conclude that the standard initial evaluation of a child with a febrile UTI should begin with a renal scan and not a VCUG (the “top-down” approach). Using this approach, only in those children with an abnormal scan should a VCUG be obtained. The benefit of such an approach would be a reduction in the number of children undergoing VCUG and the identification of reflux in a higher risk group; in theory, patients with reflux but no structural or functional renal abnormalities might go undiagnosed, but the absence of such renal abnormalities suggests that the reflux is not likely to be clinically significant. Use of ultrasound as an alternative to renal scan has not been accepted because of decreased sensitivity. One study noted that up to 25% of patients with cortical defects on DMSA had a normal ultrasound, providing further data on the utility of renal scans in the evaluation of children with febrile UTIs.72
Mercaptoacetyltriglycine (MAG3) scanning also has an improved ability to detect renal scarring compared to IVP. Although the DMSA scan is considered by many to be the most sensitive test for renal scar detection; the reported sensitivity rate of the MAG3 compared to the DMSA varies from 88% to equal to or slightly better than a DMSA scan.73,74,75 Advantages of MAG3 include lower radiation exposure, cost, and time requirement, as well as visualization of the collecting system which may improve specificity compared to DMSA in those with significant dilation of the collecting system.73 Nuclear scintigraphy is usually deferred until 4-6 weeks of age to ensure adequate renal development for accurate results.
Indications for Diagnostic Testing
The diagnosis of vesicoureteral reflux is generally made after one or more UTIs or when suspected on the basis of abnormal renal imaging. Screening for VUR has traditionally been recommended in patients with prenatally-diagnosed urinary tract dilation (UTD) because reflux has been reported in up to 31% of infants with prenatal UTD; however prenatal UTD correlates poorly with severity of VUR.76,77,78,79 The risk of VUR in patients with a non-dilated or a mildly dilated collecting system may be as high as 25%.77,80,81 Fortunately, VUR associated with prenatally detected UTD has a high incidence of spontaneous resolution.81,82 Studies that assessed whether or not patients with both UTD and VUR are at increased risk for UTI (compared to UTD in the absence of VUR) show conflicting results.83,84,85,86,87,88,89,90 Judicious performance of VCUG should be considered based on risk factors for clinically significant VUR.
The utility of sibling screening for reflux is also controversial.17,91 there is an increased risk of renal cortical abnormalities in screened siblings with history of UTI as well as ultimate diagnosis of high grade VUR.92 One study using insurance claims data did not find a significant difference in the rate of UTI between screened and unscreened siblings.93
It remains to be demonstrated whether detection and management of VUR in an asymptomatic screened sibling will result in significantly decreased adverse sequelae; therefore, no consensus regarding the practice of asymptomatic sibling screening for VUR currently exists. The AUA Clinical Guidelines for the Management of Primary Vesicoureteral Reflux in Children recommendation is that siblings be screened with a renal-bladder ultrasound (RBUS), with VCUG reserved for those in whom the RBUS is abnormal.94
Timing of evaluation for VUR in patients with history of febrile UTI also remains controversial. The AAP Subcommittee on Urinary Tract Infection published clinical guidelines in 2011 and reaffirmed in 2016 that recommended deferring antibiotic prophylaxis and VCUG after the first febrile UTI in the absence of an abnormal renal ultrasound in children aged 2-24 months.95 The Randomized Intervention in Children with Vesicoureteral Reflux (RIVUR) trial studied 607 young children (2-72 months) with grades I-IV VUR after a first or second febrile or symptomatic UTI, and randomized them to placebo versus antibiotic prophylaxis. Trimethoprim/sulfamethoxazole prophylaxis reduced the occurrence of UTI by 50%, but there was no significant difference in renal scarring between the groups, although the study was not powered to address renal scarring.96 A post hoc analysis of the RIVUR trial and another multicenter prospective trial (CUTIE) showed the incidence of renal scarring after one febrile UTI to be 2.8%, 25.7% after two febrile UTIs, and 28.6% after three or more febrile UTIs, reinforcing the utility of VUR screening after initial febrile UTI.97
Clinical Management
There is no universal optimal management for children with VUR. As previously noted, multiple anatomic and physiologic variables influence the likelihood of spontaneous VUR resolution and risk of febrile UTIs, while extrinsic factors such as patient and family preferences, medication compliance, social situations and risk of urinary tract infection rates must also be considered. Keeping in mind that the management of VUR should be individualized to each child after consideration of the multiple intrinsic and extrinsic factors which influence outcome, we discuss the various treatment options below.
Nonoperative Management
Daily administration of low-dose (~ ¼ treatment dose) antibiotics is based on the knowledge that spontaneous resolution rates for primary VUR are very high (even for severe VUR in selected populations), and that postnatal reflux-associated renal scarring appears to occur exclusively in the setting of infected urine, particularly in the poles of the kidneys where the intrarenal collecting system is more likely to have compound calyces.98 Thus, maintenance of sterile urine until spontaneous reflux resolution may avoid the morbidity of surgery and renal scarring. A number of randomized studies have attempted to evaluate the efficacy and adverse effects of antibiotic prophylaxis in children with VUR.4,99 These studies have generally failed to show a significant reduction in acute pyelonephritis or renal scars in children with VUR being treated with antibiotic prophylaxis. Some studies actually reported an increase in UTIs in children on antibiotics as well as an increase in antibiotic resistant bacteria causing the UTIs.4,99,100 By subset analysis, other studies identified younger age and increasing degrees of reflux as risk factors for recurrent febrile UTIs.99,101 The multisite RIVUR trial demonstrated a reduction in UTI recurrence by 50% with antibiotic prophylaxis vs. placebo but no difference in the rate of renal scarring.102
Many question the need for antibiotic prophylaxis, suggesting that, in select individuals, the chance for pyelonephritis and renal damage off prophylactic antibiotics is small.103,104,105,106 Between 30% and 50% of children with a history of one UTI will suffer from recurrent infections and because the diagnosis of reflux often follows a urinary tract infection, it leads many individuals to the erroneous assumption that the reflux is responsible for the infection. However, in general it does not significantly predispose to urinary tract infections unless it is of a higher grade.104,107 More often, UTIs are due to predisposing conditions such as a previous history of urinary tract infections, female gender, constipation, infrequent voiding, incomplete bladder emptying, or impaired immunity. Multiple studies now demonstrate that children on antibiotic prophylaxis without breakthrough infections or evidence of renal injury can be safely observed without antibiotic prophylaxis or correction of VUR.103,104 especially after bowel and bladder dysfunction has been optimized.108
In general, daily antibiotic prophylaxis appears to be safe and well tolerated, but it does incur cost and potential risks. It has been associated with a 24-fold increased risk of trimethoprim-sulfamethoxazole resistant Escherichia coli.109 Other studies have demonstrated the emergence of other bacteria with high rates of resistance in children receiving prophylactic antibiotics.110 In addition to resistance, there are other concerns regarding potential side effects of long term antibiotics on the gut and urinary microbiome, as well as growth.111,112,113,114 Additionally, medication adherence should be considered. A 2010 study suggested that the compliance rate for merely filling the prescription was only 40%, suggesting that many patients placed on antibiotic prophylaxis never receive the medication.115
Recognition and treatment of bladder dysfunction plays a large role in conservative management of VUR whether in isolation or with antibiotic prophylaxis. It is now recognized that secondary VUR is more appropriately managed by addressing the lower urinary tract dysfunction.116
Several large prospective studies have attempted to address the efficacy of operative intervention versus antibiotic prophylaxis. These studies have generally shown no significant difference in renal function or growth, the progression or development of new scars, or UTIs.23,36,107,117,118 However, pyelonephritic symptoms, including febrile UTIs, tended to be more common in the medically treated groups.18,105,119 In general, children who ultimately underwent surgical intervention tended to develop renal scars at an earlier age, but no significant difference occurred overall with longer follow-up in terms of new renal scars in those treated with antibiotics compared to operatively.24,120 These observations suggested that a potential benefit might be a reduction in pyelonephritis from the antireflux operations for some patients; however, other researchers suggested that, once renal scarring occurs, the disease tends to run its course and operative treatment has little benefit.27,121 One review concluded that nine ureteral reimplantation surgeries would be required to prevent one febrile UTI, with no reduction in the number of children developing renal damage,24 again reinforcing the need to better define which children with VUR may benefit from intervention. Operative intervention is generally reserved for children with breakthrough UTI while on antibiotic prophylaxis, worsening renal function, or those in whom other considerations favor definitive intervention over daily antibiotic administration.
Operative Management
Endoscopic Treatment
Since FDA approval of the use of dextranomer/hyaluronic acid copolymer (Dx/HA) (Deflux®, Q-Med, Uppsala, Sweden) in 2001 for the treatment of primary VUR, providers have increasingly used endoscopic injection as an alternative to prolonged antibiotics.122 Dx/HA is the only FDA approved commercially available injectable treatment for reflux in the United States. It is a synthetic mixture of dextran microspheres in a hyaluronic acid gel that is injected using various templates in the region of the ureteral orifices. The dextranomer particle size prevents lymphatic migration.123
Endoscopic correction of VUR offers a minimally invasive, outpatient procedure with low risk of complications. While it is a seemingly simple procedure, several studies have demonstrated a learning curve with improved results obtained with increasing experience.124,125,126 Other factors associated with successful endoscopic correction include lower reflux grade, smaller UDR, absence of bowel and bladder dysfunction, increased volume of Dx/HA injected, visual assessment of mound configuration following injection, and surgical technique.126,127,128,129 Studies of surgical technique have shown that the double hydrodistention implantation technique (HIT) enables higher success rates than other techniques including the STING technique, approaching that of ureteral reimplantation.130
In the short term, VUR resolution rates for a single ureter treated with Dx/HA range from 59% to 95%.126,131 If patients undergo a second injection for persistent VUR, the success rate is improved, but a third injection is rarely curative.122,124,132 Long-term outcomes of patients with >5 years of follow up show VUR resolution rates anywhere from 59-100%, affected significantly by degree of VUR, with rates of febrile UTI after injection ranging from 4-25%.130 Routine VCUG to confirm VUR resolution is controversial and not considered to be a requirement by some unless a patient is considered high risk for recurrence (high grade VUR, presence of febrile UTI after injection, age < 2 years) or if it is family or surgeon preference.133 Others advocate selectively obtaining VCUG only if a mound is not visualized on ultrasound. Ultrasound should be obtained post-operatively to rule out ureteral obstruction.134
Aside from treatment failure, calcification of the implant secondary to a foreign body reaction has recently been reported as another potential long-term factor. Mound calcification is not unique to Dx/HA and has been described with many endoscopically injected agents.135,136 Calcification can be confused with ureterolithiasis, and providers must maintain a high level of suspicion for this entity to avoid unnecessary diagnostic tests and surgical interventions.137 Ureteral obstruction following Dx/HA is reported infrequently (< 1%) and can occur shortly after surgery but has also been reported up to five years postoperatively, underscoring the importance of continued radiographic follow up of patients following Deflux. Obstruction can be asymptomatic, present with renal colic or urinary tract infection.138,139 Obstruction typically requires ureteral reimplantation with excision of the affected segment.139,140,141
Ureteral Reimplantation
Surgical treatment of vesicoureteral reflux has evolved over the last five decades. A lower abdominal transverse incision for an open technique is typically used, leaving a small scar in the skin crease that is inconspicuous. Numerous operative techniques for ureteral reimplantation have been utilized; the primary differences include intravesical (Figure 8) vs. extravesical approach. There is no clear documentation of any of particular technique being superior,23 and selection of a given technique is typically individualized to the child at the discretion of the operating physician.
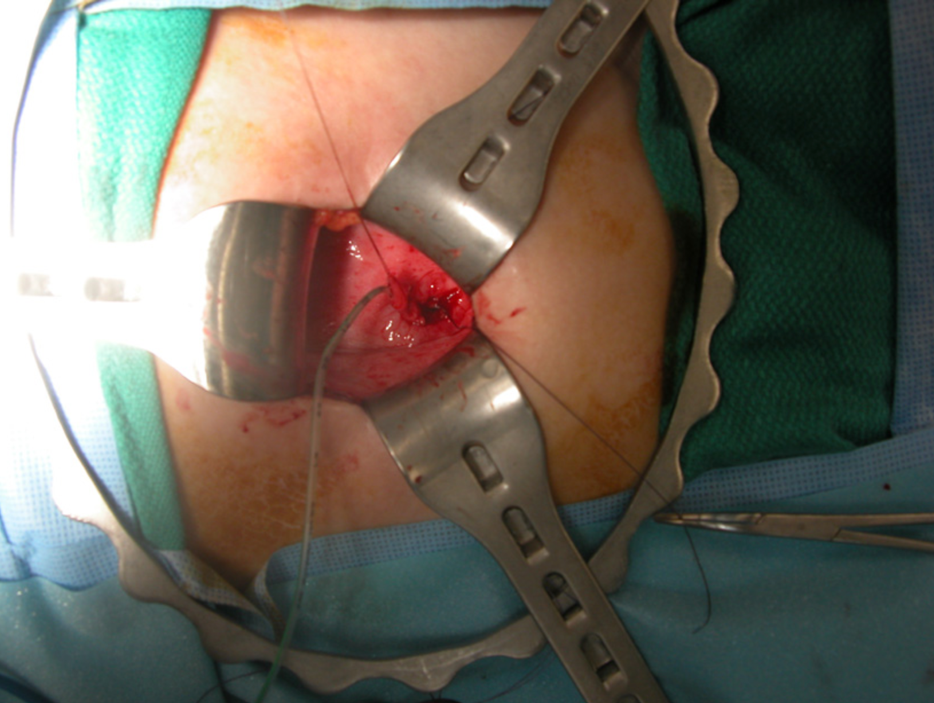
Figure 8 Intravesical ureteral reimplantation
Advancements in analgesia, surgical techniques, and the understanding that children undergoing ureteral reimplantation for primary VUR rarely need ureteral stents or prolonged bladder drainage has improved the length of stay and decreased the morbidity of the procedure.142 Several series report patients undergoing both intravesical and extravesical ureteral reimplantation surgery as outpatients.143,144,145 Results of multiple series document success rates with open ureteral reimplantation of greater than 95% and close to 100% for lower grades of reflux.146,147,148 The procedures do carry risks of anesthesia and potential complications including ureteral obstruction, persistent reflux, infection and bleeding.23 General tenets of ureteral reimplantation include minimizing ureteral handling, excision of the intravesical ureteral segment, development of a tunnel at least five times the diameter of the ureteral lumen, and creation of a tension-free anastomosis. In large ureters, tailoring through either excision or plication may be necessary to facilitate achievement of an adequate intravesical tunnel.
In recent years robotic-assisted laparoscopic extravesical ureteral reimplant has been increasingly used, with cited benefits of decreased pain and shorter postoperative stay.149 One multi-institutional study of 260 patients showed radiographic resolution of VUR in 88% of patients, a 9.6% overall complication rate and 4% rate of urinary retention following bilateral reimplant.150 A nationwide data analysis showed increased rates of complications (13% vs. 4.5%) and increased costs compared to open procedures.151 Although use of robotic ureteral reimplantation continues as some centers, the use of open surgery remains the most prevalent.152
In patients in whom surgical intervention is considered, timing of elective surgery remains a matter of debate. Most studies evaluating spontaneous resolution rates for VUR have followed patients for 5 years. Although this endpoint is presumably based on the fact that the likelihood of de novo reflux nephropathy declines after age five,153 many clinicians and families infer that children who have failed to resolve their reflux by that time should undergo corrective surgery. However, in children without infections, timing of surgery can often be delayed; ureteral reimplantations in post-pubertal children and adults have been reported, with modifications in surgical technique owing to the differences in body habitus between adults and children, but with generally good outcomes.154
Individualized Management
While VUR itself can be simply defined, more detailed research confirms that reflux is not a single condition but rather occurs with wide variations in severity and impact. The answers to many questions regarding reflux remain unknown; however, it is clear that definitive treatment and even diagnosis of VUR is of questionable clinical benefit for many patients. Currently, decisions regarding treatment are based on physician and parent assessment of risks and benefits. Although the decision to pursue surgery has traditionally been based predominantly on the grade of reflux, a truly informed decision must consider multiple other variables, such as patient age, gender, history of UTI, renal functional status, likeliness and timing of spontaneous resolution, and risk of subsequent febrile UTI. Multiple prognostic factors relative to a child’s chance for spontaneous reflux resolution have been defined.38,47,50,65 A patient’s social situation and parental preferences, as well as willingness to comply with either conservative management or postoperative care, must also be taken into consideration.
Attempting to determine the likelihood and timing of spontaneous VUR resolution in a particular child while taking into account multiple prognostic variables such as age, gender, grade of VUR, ureteral diameter ratio (UDR), bladder volume at onset of reflux, presence of dysfunctional voiding, history of UTIs, presence of renal scarring, laterality and duplication is extremely complex. To this end, a user-friendly neural network that incorporates many of these predictive factors is available for use at http://pedsurocomp.lab.uiowa.edu (Figure 9)155
Figure 9 Neural network incorporating predictive factors for resolution of VUR
The use and accuracy of this model was validated on an international basis in a group of Japanese children.66 For children who have had a renal scan, a second computer model was generated incorporating the additional renal scan data to improve prognostic accuracy and is available at the same website (http://pedsurocomp.lab.uiowa.edu).50
Future Directions
Although improved accuracy regarding the likelihood and timing of spontaneous VUR resolution permits better management decisions, additional data is needed. Further studies are needed to define an individual child’s risk of subsequent pyelonephritis, renal damage, and ultimately clinical sequelae. Continued data collection from large, multi-institutional prospective registries can allow for better understanding of the natural history of VUR. Additionally, biomarkers for detection of clinically meaningful vesicoureteral reflux may allow us to better determine those who most benefit from active treatment.
Key Points
- Although the International Reflux Grading System is predictive for spontaneous resolution of VUR and for presence of renal scarring, it has poor interrater reliability. More objective radiographic measures in addition to grade such as distal ureteral diameter ratio and bladder volume at onset of VUR are predictive for spontaneous resolution and risk of breakthrough UTI.
- Multiple clinical and radiographic factors found to be predictive of clinical VUR outcomes are used in a variety of combinations in risk calculators and user-friendly neural networks such as http://pedsurocomp.lab.uiowa.edu to permit a more accurate individualized risk assessment in order to better manage patients.
- Children with renal scarring associated with VUR are more likely to develop hypertension, proteinuria and CKD and should undergo routine surveillance of blood pressure, renal function studies and urinalysis for proteinuria through adulthood.
- VCUG based on the presence of prenatal urinary tract dilation alone is often not clinically useful and a shared decision-making approach with the parents that incorporates the risk for UTI based on additional clinical and sonographic factors (i.e. severity of hydronephrosis, presence of hydroureter) is recommended to determine if a child should undergo VCUG
- Adequate control of bowel and bladder dysfunction plays a crucial role in the conservative management of vesicoureteral reflux with or without the use of antibiotic prophylaxis, and can in some cases obviate the need for surgical intervention.
- Aside from the development of antibiotic resistant bacteria, chronic antibiotic prophylaxis may have long-term side-effects including adverse impacts on the normal gut and urinary microbiome which may influence childhood growth and development.
References
- Mathews R, Carpenter M, Chesney R. Controversies in the management of vesicoureteral reflux: the rationale for the RIVUR study. J Pediatr Urol 2009; 5 (5): 336–341. DOI: 10.1016/J.JPUROL.2009.05.010.
- Kaefer M, Curran M, Treves ST. Sibling vesicoureteral reflux in multiple gestation births. Pediatrics 2000; 105 (4 Pt 1): 800–804. DOI: 10.1542/PEDS.105.4.800.
- Smellie JM, Poulton A, Prescod NP. Retrospective study of children with renal scarring associated with reflux and urinary infection. 1994; 308 (6938): 1193–1196. DOI: 10.1136/bmj.308.6938.1193.
- Garin EH, Olavarria F, Nieto VG. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics 2006; 117 (3): 626–632. DOI: 10.1542/PEDS.2005-1362.
- Cooper CS, Austin JC. Vesicoureteral reflux: who benefits from surgery? Urol Clin North Am 2004; 31 (3): 535–541. DOI: 10.1016/j.ucl.2004.04.006.
- Mattoo TK. Vesicoureteral reflux and reflux nephropathy. Adv Chronic Kidney Dis 2011; 18 (5): 348–354. DOI: 10.1053/j.ackd.2011.07.006.
- Cornwell LB, Riddell JV, Mason MD. New-onset ESRD secondary to reflux nephropathy has decreased in incidence in the United States. J Pediatr Urol 2020; 16 (5). DOI: 10.1016/j.jpurol.2020.06.023.
- Zhang Y, Bailey RR. A long term follow up of adults with reflux nephropathy. N Z Med J 1995; 108 (998): 7761049.
- Hutch JA. Vesico-ureteral reflux in the paraplegic: cause and correction. J Urol 1952; 68 (2): 457–469. DOI: 10.1016/s0022-5347(05)65331-1.
- Hodson CJ. The radiological diagnosis of pyelonephritis. Proc R Soc Med 1959; 52 (8): 669–672.
- Kalayeh K, Fowlkes JB, Schultz WW. Ureterovesical junction deformation during urine storage in the bladder and the effect on vesicoureteral reflux. Journal of Biomechanics 2020. DOI: 10.1016/J.JBIOMECH.2020.110123.
- Paquin AJ. Ureterovesical anastomosis: the description and evaluation of a technique. J Urol 1959; 82 (5): 573–583. DOI: 10.1016/S0022-5347(17)65934-2.
- Koff SA. Relationship between dysfunctional voiding and reflux. J Urol 1992. DOI: 10.1016/s0022-5347(17)37007-6.
- AM E, K D, E R. Genes in the ureteric budding pathway: association study on vesico-ureteral reflux patients. PLoS ONE 2012; 7 (4). DOI: 10.1371/journal.pone.0031327.
- Noe HN. The long-term results of prospective sibling reflux screening. J Urol 1992. DOI: 10.1016/s0022-5347(17)37017-9.
- Wan J, Greenfield SP, Ng M. Sibling reflux: a dual center retrospective study. J Urol 1996; 156 (2 Pt 2): 8683758. DOI: 10.1016/s0022-5347(01)65782-3.
- Noe HN. The relationship of sibling reflux to index patient dysfunctional voiding. J Urol 1988; 140 (1): 3379674. DOI: 10.1016/s0022-5347(17)41502-3.
- Weiss R, Duckett J, Spitzer A. Results of a randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (United States. The International Reflux Study in Children J Urol 1992. DOI: 10.1016/s0022-5347(17)36998-7.
- Smellie JM. Reflections on 30 years of treating children with urinary tract infections. J Urol 1991; 146 (2 ( Pt 2): 10 1016 0022–5347 17 37889–37888. DOI: 10.1016/s0022-5347(17)37889-8.
- Nguyen HT, Bauer SB, Peters CA. 99m Technetium dimercapto-succinic acid renal scintigraphy abnormalities in infants with sterile high grade vesicoureteral reflux. J Urol 2000; 164 (5): 10 1016 0022–5347 05 67081–67084. DOI: 10.1097/00005392-200011000-00076.
- Patterson LT, Strife CF. Acquired versus congenital renal scarring after childhood urinary tract infection. J Pediatr 2000; 136 (1): 2–4. DOI: 10.1016/s0022-3476(00)90038-6.
- Bailey RR, Lynn KL, Smith AH. Long-term followup of infants with gross vesicoureteral reflux. J Urol 1992. DOI: 10.1016/s0022-5347(17)37010-6.
- Elder JS, Peters CA, Arant BS Jr.. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol 1997; 157 (5): 9112544. DOI: 10.1097/00005392-199705000-00093.
- Hodson EM, Wheeler DM, Vimalchandra D. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev 2007 (3). DOI: 10.1002/14651858.CD001532.pub3.
- Baker R, Maxted W, Maylath J. Relation of age, sex, and infection to reflux: Data indicating high spontaneous cure rate in pediatric patients. J Urol 1966; 95 (1): 10 1016 0022–5347 17 63403–63407. DOI: 10.1016/s0022-5347(17)63403-7.
- Ransley PG, Risdon RA. Reflux nephropathy: effects of antimicrobial therapy on the evolution of the early pyelonephritic scar. Kidney International 1981; 20 (6): 733–742. DOI: 10.1038/KI.1981.204.
- Winberg J. Management of primary vesico-ureteric reflux in children\–operation ineffective in preventing progressive renal damage. Infection 1994; 22 Suppl 1:S4-7. DOI: 10.1007/bf01716025.
- Sukamoto E, Itoh K, Morita K. Reappraisal of Tc-99m DMSA scintigraphy for follow up in children with vesicoureteral reflux. Ann Nucl Med 1999; 13 (6): 401–406. DOI: 10.1007/bf03164934.
- Naseer S SR, G.F.. New renal scars in children with urinary tract infections, vesicoureteral reflux and voiding dysfunction: a prospective evaluation. J Urol 1997; 158 (2): 9224361. DOI: 10.1016/s0022-5347(01)64552-x.
- Gordon I, Barkovics M, Pindoria S. Primary vesicoureteric reflux as a predictor of renal damage in children hospitalized with urinary tract infection: a systematic review and meta-analysis. J Am Soc Nephrol 2003; 14 (3): 739–744. DOI: 10.1097/01.asn.0000053416.93518.63.
- Lebowitz RL, Olbing H, Parkkulainen KV. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children Pediatr Radiol 1985; 15 (2): 105–109. DOI: 10.1007/bf02388714.
- Schwab CW Jr., Wu HY, Selman H. Spontaneous resolution of vesicoureteral reflux: a 15-year perspective. J Urol 2002; 168 (6): 2594–2599. DOI: 10.1016/S0022-5347(05)64225-5.
- Hoberman A, Charron M, Hickey RW. Imaging Studies after a First Febrile Urinary Tract Infection in Young Children. New Engl J Med 2003; 348 (3): 195–202. DOI: 10.1056/NEJMoa021698.
- Ylinen E, Ala-Houhala M, Wikström S. Risk of renal scarring in vesicoureteral reflux detected either antenatally or during the neonatal period. Urology 2003; 61 (6): 1242–1243. DOI: 10.1016/s0090-4295(03)00229-2.
- Rolleston GL, Shannon FT, Utley WL. Relationship of infantile vesicoureteric reflux to renal damage. Br Med J 1970; 1 (5694): 460–463. DOI: 10.1136/bmj.1.5694.460.
- Duckett JW, Walker RD, Weiss R. Surgical results: International Reflux Study in Children\–United States branch. J Urol 1992. DOI: 10.1016/s0022-5347(17)36999-9.
- Berg UB. Long-term followup of renal morphology and function in children with recurrent pyelonephritis. J Urol 1992. DOI: 10.1016/s0022-5347(17)37012-x.
- Nepple KG, Knudson MJ, Austin JC. Abnormal renal scans and decreased early resolution of low grade vesicoureteral reflux. Suppl):1643-7; Discussion 1647 2008; 180(4. DOI: 10.1016/j.juro.2008.03.102.
- Metcalfe CB, Macneily AE, Afshar K. Reliability assessment of international grading system for vesicoureteral reflux. J Urol 2012; 188(4. DOI: 10.1016/j.juro.2012.02.015.
- Greenfield SP, Carpenter MA, Chesney RW. The RIVUR voiding cystourethrogram pilot study: experience with radiologic reading concordance. J Urol 2012; 188(4. DOI: 10.1016/j.juro.2012.06.032.
- Cooper CS, Alexander SE, Kieran K. Utility of the distal ureteral diameter on VCUG for grading VUR. J Pediatr Urol 2015; 11 (4). DOI: 10.1016/j.jpurol.2015.04.009.
- Arlen AM, Leong T, Guidos PJ. Distal Ureteral Diameter Ratio is Predictive of Breakthrough Febrile Urinary Tract Infection. J Urol 2017; 198 (6): 1418–1423. DOI: 10.1016/j.juro.2017.06.095.
- Cooper CS, Birusingh KK, Austin JC. Distal ureteral diameter measurement objectively predicts vesicoureteral reflux outcome. J Pediatr Urol 2013; 9 (1): 99–103. DOI: 10.1016/j.jpurol.2011.12.011.
- Arlen AM, Kirsch AJ, Leong T. Validation of the ureteral diameter ratio for predicting early spontaneous resolution of primary vesicoureteral reflux. J Pediatr Urol 2017; 13 (4). DOI: 10.1016/j.jpurol.2017.01.012.
- Troesch VL, Wald M, Bonnett MA. The additive impact of the distal ureteral diameter ratio in predicting early breakthrough urinary tract infections in children with vesicoureteral reflux. J Pediatr Urol 2021; 17 (2). DOI: 10.1016/j.jpurol.2021.01.003.
- Alexander SE, Arlen AM, Storm DW. Bladder volume at onset of vesicoureteral reflux is an independent risk factor for breakthrough febrile urinary tract infection. J Urol 2015; 193 (4): 1342–1346. DOI: 10.1016/j.juro.2014.10.002.
- McMillan ZM, Austin JC, Knudson MJ. Bladder volume at onset of reflux on initial cystogram predicts spontaneous resolution. J Urol 2006. DOI: 10.1016/s0022-5347(06)00619-7.
- Nepple KG, Knudson MJ, Austin JC. Adding renal scan data improves the accuracy of a computational model to predict vesicoureteral reflux resolution. Suppl):1648-52; Discussion 1652 DOI: 101016/Jjuro200803109 PMID 2008; 180(4: 1648–1652. DOI: 10.1016/j.juro.2008.03.109.
- Knudson MJ, Austin JC, Wald M. Computational model for predicting the chance of early resolution in children with vesicoureteral reflux. Pt 2):1824-7 DOI: 101016/Jjuro200705093 PMID 2007; 178: 1824–1827. DOI: 10.1016/j.juro.2007.05.093.
- Knudson MJ, Austin JC, McMillan ZM. Predictive factors of early spontaneous resolution in children with primary vesicoureteral reflux. J Urol 2007; 178(4. DOI: 10.1016/j.juro.2007.03.161.
- KJ A, MT M, JC A. Nuclear cystometrogram-determined bladder pressure at onset of vesicoureteral reflux predicts spontaneous resolution. Urology 2007; 69 (4): 767–770. DOI: 10.1016/j.urology.2007.01.048.
- Cooper CS, Madsen MT, Austin JC. Bladder pressure at the onset of vesicoureteral reflux determined by nuclear cystometrogram. J Urol 2003; 170: 1537–1540. DOI: 10.1097/01.ju.0000083638.36182.5e.
- Arsanjani A, Alagiri M. Identification of filling versus voiding reflux as predictor of clinical outcome. Urology 2007; 70 (2): 351–354. DOI: 10.1016/j.urology.2007.03.031.
- Garcia-Roig M, Ridley DE, McCracken C. Vesicoureteral Reflux Index: Predicting Primary Vesicoureteral Reflux Resolution in Children Diagnosed after Age 24 Months. J Urol 2017; 197 (4): 1150–1157. DOI: 10.1016/j.juro.2016.12.008.
- Arlen AM, Leong T, Wu CQ. Predicting Breakthrough Urinary Tract Infection: Comparative Analysis of Vesicoureteral Reflux Index, Reflux Grade and Ureteral Diameter Ratio. J Urol 2020; 204 (3): 572–577. DOI: 10.1097/JU.0000000000001035.
- Keren R, Shaikh N, Pohl H. Risk Factors for Recurrent Urinary Tract Infection and Renal Scarring. Pediatrics 2015; 136 (1). DOI: 10.1542/peds.2015-0409.
- Lenaghan D, Whitaker JG, Jensen F. The natural history of reflux and long-term effects of reflux on the kidney. J Urol 1976; 115 (6): 10 1016 0022–5347 17 59352–59350. DOI: 10.1016/s0022-5347(17)59352-0.
- Olbing H, Claësson I, Ebel KD. Renal scars and parenchymal thinning in children with vesicoureteral reflux: a 5-year report of the International Reflux Study in Children (European branch. J Urol 1992. DOI: 10.1016/s0022-5347(17)36995-1.
- Mingin GC, Nguyen HT, Baskin LS. Abnormal dimercapto-succinic acid scans predict an increased risk of breakthrough infection in children with vesicoureteral reflux. J Urol 2004; 172 (3): 1075–1077. DOI: 10.1097/01.ju.0000135750.17348.e4.
- Loukogeorgakis SP, Burnand K, MacDonald A. Renal scarring is the most significant predictor of breakthrough febrile urinary tract infection in patients with simplex and duplex primary vesico-ureteral reflux. J Pediatr Urol 2020; 16 (2). DOI: 10.1016/j.jpurol.2019.11.018.
- Wallace DM, Rothwell DL, Williams DI. The long-term follow-up of surgically treated vesicoureteric reflux. Br J Urol 1978; 50 (7): 10 1111 1464–1410 1978 06195. DOI: 10.1111/j.1464-410x.1978.tb06195.x.
- Edwards D, Normand IC, Prescod N. Disappearance of vesicoureteric reflux during long-term prophylaxis of urinary tract infection in children. Br Med J 1977; 2 (6082): 285–288. DOI: 10.1136/bmj.2.6082.285.
- Jodal U, Lindberg U. Guidelines for management of children with urinary tract infection and vesico-ureteric reflux. Recommendations from a Swedish state-of-the-art conference. Swedish Medical Research Council Acta Paediatr Suppl 1999; 88 (431): 87–89. DOI: 10.1111/j.1651-2227.1999.tb01323.x.
- Mor Y, Leibovitch I, Zalts R. Analysis of the long-term outcome of surgically corrected vesico-ureteric reflux. BJU Int 2003; 92 (1): 10 1046 1464–1410 2003 04264. DOI: 10.1046/j.1464-410x.2003.04264.x.
- Lebowitz RL. The detection and characterization of vesicoureteral reflux in the child. J Urol 1992. DOI: 10.1016/s0022-5347(17)36991-4.
- Dalirani R, Mahyar A, Sharifian M. The value of direct radionuclide cystography in the detection of vesicoureteral reflux in children with normal voiding cystourethrography. Pediatr Nephrol 2014; 29 (12): 10 1007 00467–00014–2871–. DOI: 10.1007/s00467-014-2871-y.
- McLaren CJ, Simpson ET. Direct comparison of radiology and nuclear medicine cystograms in young infants with vesico-ureteric reflux. BJU Int 2001; 87 (1): 10 1046 1464–1410 2001 00997. DOI: 10.1046/j.1464-410x.2001.00997.x.
- Frimberger D, Mercado-Deane MG, McKenna PH. Establishing a Standard Protocol for the Voiding Cystourethrography. Pediatrics 2016; 138 (5): 10 1542 2016–2590. DOI: 10.1542/9781610021494-part05-establishing_a_stand.
- Strauss KJ, Kaste SC. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients\–a white paper executive summary. Radiology 2006; 240 (3): 621–622. DOI: 10.1148/radiol.2403060698.
- Arant BS Jr. Vesicoureteral reflux and evidence-based management. J Pediatr 2001; 139 (5): 620–621. DOI: 10.1067/mpd.2001.119451.
- Shiraishi K, Matsuyama H, Nepple KG. Validation of a prognostic calculator for prediction of early vesicoureteral reflux resolution in children. J Urol 2009; 182 (2): 690–691. DOI: 10.1016/j.juro.2009.04.036.
- Kim D, Choi YH, Choi G. Contrast-enhanced voiding urosonography for the diagnosis of vesicoureteral reflux and intrarenal reflux: a comparison of diagnostic performance with fluoroscopic voiding cystourethrography. Ultrasonography 2021; 40 (4): 530–537. DOI: 10.14366/usg.20157.
- Ntoulia A, Back SJ, Shellikeri S. Contrast-enhanced voiding urosonography (ceVUS) with the intravesical administration of the ultrasound contrast agent OptisonTM for vesicoureteral reflux detection in children: a prospective clinical trial. Pediatr Radiol 2018; 48 (2): 10 1007 00247–00017–4026–4023. DOI: 10.1007/s00247-017-4026-3.
- Elison BS, Taylor D, Wall H. Comparison of DMSA scintigraphy with intravenous urography for the detection of renal scarring and its correlation with vesicoureteric reflux. Br J Urol 1992; 69 (3): 10 1111 1464–1410 1992 15532. DOI: 10.1111/j.1464-410x.1992.tb15532.x.
- Majd M, Nussbaum Blask AR, Markle BM. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology 2001; 218 (1): 101–108. DOI: 10.1148/radiology.218.1.r01ja37101.
- Hamoui N, Hagerty JA, Maizels M. Ultrasound fails to delineate significant renal pathology in children with urinary tract infections: a case for dimercapto-succinic acid scintigraphy. Suppl):1639-42; Discussion 1642 2008; 180(4. DOI: 10.1016/j.juro.2008.03.119.
- Smokvina A, Grbac-Ivanković S, Girotto N. The renal parenchyma evaluation: MAG3 vs. DMSA Coll Antropol 2005; 29 (2): 649–654.
- Sfakianakis GN, Cavagnaro F, Zilleruelo G. Diuretic MAG3 scintigraphy (F0) in acute pyelonephritis: regional parenchymal dysfunction and comparison with DMSA. J Nucl Med 2000; 41 (12): 1955–1963.
- Gordon I, Anderson PJ, Lythgoe MF. Can technetium-99m-mercaptoacetyltriglycine replace technetium-99m-dimercaptosuccinic acid in the exclusion of a focal renal defect? J Nucl Med 1992; 33 (12): 1334134.
- Lee RS, Cendron M, Kinnamon DD. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics 2006; 118 (2): 586–593. DOI: 10.1542/PEDS.2006-0120.
- Herndon CDA, McKenna PH, Kolon TF. A multicenter outcomes analysis of patients with neonatal reflux presenting with prenatal hydronephrosis. J Urol 1999; 162 (3 Pt 2): 1203–1208. DOI: 10.1097/00005392-199909000-00096.
- Nguyen HT, Herndon CDA, Cooper C. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol 2010; 6 (3): 212–231. DOI: 10.1016/J.JPUROL.2010.02.205.
- Upadhyay J, McLorie GA, Bolduc S. Natural history of neonatal reflux associated with prenatal hydronephrosis: Long-term results of a prospective study. J Urol 2003; 169 (5): 1837–1841. DOI: 10.1097/01.ju.0000062440.92454.cf.
- Berrocal T, Pinilla I, Gutiérrez J. Mild hydronephrosis in newborns and infants: can ultrasound predict the presence of vesicoureteral reflux. Pediatr Nephrol 2007; 22 (1): 91–96. DOI: 10.1007/S00467-006-0285-1.
- Farhat W, McLorie G, Geary D. The natural history of neonatal vesicoureteral reflux associated with antenatal hydronephrosis. J Urol 2000; 164 (3 Pt 2): 1057–1060. DOI: 10.1097/00005392-200009020-00033.
- M A, K W-L, BK V. Society for fetal urology recommendations for postnatal evaluation of prenatal hydronephrosis\–will fewer voiding cystourethrograms lead to more urinary tract infections? J Urol 2013. DOI: 10.1016/J.JURO.2013.03.038.
- Braga LH, Farrokhyar F, DĆruz J. Risk factors for febrile urinary tract infection in children with prenatal hydronephrosis: a prospective study. J Urol 2015. DOI: 10.1016/J.JURO.2014.10.091.
- Zee RS, Herndon CDA, Cooper CS. Time to resolution: A prospective evaluation from the Society for Fetal Urology hydronephrosis registry. J Pediatr Urol 2017; 13 (3). DOI: 10.1016/J.JPUROL.2016.12.012.
- Silay MS, Undre S, Nambiar AK. Role of antibiotic prophylaxis in antenatal hydronephrosis: A systematic review from the European Association of Urology/European Society for Paediatric Urology Guidelines Panel. J Pediatr Urol 2017; 13 (3): 306–315. DOI: 10.1016/J.JPUROL.2017.02.023.
- Braga LH, Mijovic H, Farrokhyar F. Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics 2013; 131 (1): 10 1542 2012–1870. DOI: 10.1542/peds.2012-1870.
- Easterbrook B, Capolicchio JP, Braga LH. Antibiotic prophylaxis for prevention of urinary tract infections in prenatal hydronephrosis: An updated systematic review. Can Urol Assoc J 2017; 11 (1-2Suppl1). DOI: 10.5489/CUAJ.4384.
- Zareba P, Lorenzo AJ, Braga LH. Risk factors for febrile urinary tract infection in infants with prenatal hydronephrosis: comprehensive single center analysis. J Urol 2014. DOI: 10.1016/J.JURO.2013.10.035.
- Coelho GM, Bouzada MCF, Pereira AK. Outcome of isolated antenatal hydronephrosis: a prospective cohort study. Pediatr Nephrology 2007; 22 (10): 1727–1734. DOI: 10.1007/S00467-007-0539-6.
- Szymanski KM, Al-Said AN, Pippi Salle JL. Do infants with mild prenatal hydronephrosis benefit from screening for vesicoureteral reflux? J Urol 2012; 188 (2): 576–581. DOI: 10.1016/J.JURO.2012.04.017.
- Routh JC, Grant FD, Kokorowski P. Costs and consequences of universal sibling screening for vesicoureteral reflux: decision analysis. Pediatrics 2010; 126 (5): 10 1542 2010–0744. DOI: 10.1542/peds.2010-0744d.
- Hunziker M, Colhoun E, Puri P. Renal cortical abnormalities in siblings of index patients with vesicoureteral reflux. Pediatrics 2014; 133 (4): 10 1542 2013–3498. DOI: 10.1542/peds.2013-3498d.
- Nelson CP, Finkelstein JA, Logvinenko T. Incidence of Urinary Tract Infection Among Siblings of Children With Vesicoureteral Reflux. Acad Pediatr 2016; 16 (5): 489–495. DOI: 10.1016/j.acap.2015.11.003.
- Skoog SJ, Peters CA, Arant BS Jr.. Pediatric Vesicoureteral Reflux Guidelines Panel Summary Report: Clinical Practice Guidelines for Screening Siblings of Children With Vesicoureteral Reflux and Neonates/Infants With Prenatal Hydronephrosis. J Urol 2010; 184 (3): 1145–1151. DOI: 10.1016/j.juro.2010.05.066.
- Urinary Tract Infection SCoQI S, Management RKB. Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics 2011; 128 (3): 595–610. DOI: 10.1542/peds.2011-1330.
- Mattoo TK, Carpenter MA, Moxey-Mims M. The RIVUR trial: a factual interpretation of our data. Pediatr Nephrol 2015; 30 (5): 10 1007 00467–00014–3022–3021. DOI: 10.1007/s00467-014-3022-1.
- Shaikh N, Haralam MA, Kurs-Lasky M. Association of Renal Scarring With Number of Febrile Urinary Tract Infections in Children. JAMA Pediatrics 2019; 173 (10): 949–952. DOI: 10.1001/JAMAPediatrics.2019.2504.
- Coulthard MG, Flecknell P, Orr H. Renal scarring caused by vesicoureteric reflux and urinary infection: a study in pigs. Pediatr Nephrol 2002; 17 (7): 10 1007 00467–00002–0878–0872. DOI: 10.1007/s00467-002-0878-2.
- Montini G, Rigon L, Zucchetta P. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics 2008; 122 (5): 1064–1071. DOI: 10.1542/peds.2007-3770.
- Pennesi M, Travan L, Peratoner L. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 2008; 121 (6): 10 1542 2007–2652. DOI: 10.1542/peds.2008-2339.
- Roussey-Kesler G, Gadjos V, Idres N. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: Results from a prospective randomized study. J Urol 2008; 179 (2): 674–679. DOI: 10.1016/J.JURO.2007.09.090.
- Conway PH, Cnaan A, Zaoutis T. Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials. JAMA 2007; 298 (2): 179–186. DOI: 10.1001/jama.298.2.179.
- Investigators RT, Hoberman A, Greenfield SP. Antimicrobial Prophylaxis for Children with Vesicoureteral Reflux. New Engl J Med 2014; 370 (25): 2367–2376. DOI: 10.1056/NEJMoa1401811.
- Cooper CS, Chung BI, Kirsch AJ. The outcome of stopping prophylactic antibiotics in older children with vesicoureteral reflux. J Urol 2000; 163 (1): 269–273. DOI: 10.1016/S0022-5347(05)68034-2.
- Thompson RH, Chen JJ, Pugach J. Cessation of prophylactic antibiotics for managing persistent vesicoureteral reflux. J Urol 2001; 166 (4): 1465–1469. DOI: 10.1097/00005392-200110000-00072.
- Jodal U, Koskimies O, Hanson E. Infection pattern in children with vesicoureteral reflux randomly allocated to operation or long-term antibacterial prophylaxis. The International Reflux Study in Children. J Urol 1992. DOI: 10.1016/s0022-5347(17)36994-x.
- Bailey RR. Commentary: the management of grades I and II (nondilating) vesicoureteral reflux. J Urol 1992. DOI: 10.1016/s0022-5347(17)37004-0.
- A prospective trial of operative versus non-operative treatment of severe vesico-ureteric reflux: 2 years\’observation in 96 children. The Birmingham Reflux Study Group. Contrib Nephrol 1984; 39 (169-85): 6744870. DOI: 10.1136/bmj.295.6592.237.
- Leslie B, Moore K, Salle JL. Outcome of antibiotic prophylaxis discontinuation in patients with persistent vesicoureteral reflux initially presenting with febrile urinary tract infection: time to event analysis. J Urol 2010; 184 (3): 1093–1098. DOI: 10.1016/j.juro.2010.05.013.
- Allen UD, MacDonald N, Fuite L. Risk factors for resistance to f́irst-line\’antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ 1999; 160 (10): 1436–1440.
- Cheng CH, Tsai MH, Huang YC. Antibiotic Resistance Patterns of Community-Acquired Urinary Tract Infections in Children With Vesicoureteral Reflux Receiving Prophylactic Antibiotic Therapy. Pediatrics 2008; 122 (6): 1212–1217. DOI: 10.1542/peds.2007-2926.
- Cooper CS. Fat, demented and stupid: An unrecognized legacy of pediatric urology? J Pediatr Urol 2017; 13 (4): 341–344. DOI: 10.1016/J.JPUROL.2017.04.027.
- Guidos PJ, Arlen AM, Leong T. Impact of continuous low-dose antibiotic prophylaxis on growth in children with vesicoureteral reflux. J Pediatr Urol 2018; 14 (4). DOI: 10.1016/J.JPUROL.2018.07.007.
- Gaither TW, Cooper CS, Kornberg Z. Predictors of becoming overweight among pediatric patients at risk for urinary tract infections. J Pediatr Urol 2019; 15 (1). DOI: 10.1016/J.JPUROL.2018.09.002.
- Akagawa Y, Kimata T, Akagawa S. Impact of Long-Term Low Dose Antibiotic Prophylaxis on Gut Microbiota in Children. J Urol 2020; 204 (6): 1320–1325. DOI: 10.1097/JU.0000000000001227.
- Copp HL, Nelson CP, Shortliffe LD. Compliance with antibiotic prophylaxis in children with vesicoureteral reflux: results from a national pharmacy claims database. J Urol 2010; 183 (5): 1994–2000. DOI: 10.1016/J.JURO.2010.01.036.
- Fast AM, Nees SN, Batavia JP. Outcomes of targeted treatment for vesicoureteral reflux in children with nonneurogenic lower urinary tract dysfunction. J Urol 2013; 190 (3): 1028–1032. DOI: 10.1016/j.juro.2013.03.005.
- Smellie JM. Commentary: management of children with severe vesicoureteral reflux. J Urol 1992. DOI: 10.1016/s0022-5347(17)37000-3.
- J BM, editor. Prospective trial of operative versus non-operative treatment of severe vesicoureteric reflux in children: five years\’observation. Birmingham Reflux Study Group. 1987; 295 (6592): 237–241. DOI: 10.1136/bmj.295.6592.237.
- Elo J, Tallgren LG, Alfthan O. Character of urinary tract infections and pyelonephritic renal scarring after antireflux surgery. J Urol 1983; 129 (2): 6834504. DOI: 10.1016/s0022-5347(17)52089-3.
- Belman AB. Vesicoureteral reflux. Pediatr Clin North Am 1997; 44 (5): 9326957. DOI: 10.53347/rid-12076.
- Ransley PG, Risdon RA. The pathogenesis of reflux nephropathy. Contrib Nephrol 1979; 16: 90–97. DOI: 10.1159/000402880.
- Molitierno JA, Scherz HC, Kirsch AJ. Endoscopic treatment of vesicoureteral reflux using dextranomer hyaluronic acid copolymer. J Pediatr Urol 2008; 4 (3): 221–228. DOI: 10.1016/j.jpurol.2007.11.015.
- Stenberg AM, Sundin A, Larsson BS. Lack of distant migration after injection of a 125iodine labeled dextranomer based implant into the rabbit bladder. J Urol 1997; 158 (5): 10 1016 0022–5347 01 64185–64185. DOI: 10.1016/s0022-5347(01)64185-5.
- Läckgren G, Wåhlin N, Sköldenberg E. Long-term followup of children treated with dextranomer/hyaluronic acid copolymer for vesicoureteral reflux. J Urol 2001; 166 (5): 10 1016 0022–5347 05 65713–65718. DOI: 10.1097/00005392-200111000-00076.
- Kirsch AJ, Perez-Brayfield MR, Scherz HC. Minimally invasive treatment of vesicoureteral reflux with endoscopic injection of dextranomer/hyaluronic acid copolymer: the Childrenś Hospitals of Atlanta experience. J Urol 2003; 170 (1): 211–215. DOI: 10.1097/01.ju.0000072523.43060.a0.
- Dave S, Lorenzo AJ, Khoury AE. Learning from the learning curve: factors associated with successful endoscopic correction of vesicoureteral reflux using dextranomer/hyaluronic acid copolymer. J Urol 2008; 180(4: 1594–1599. DOI: 10.1016/j.juro.2008.03.084.
- Kirsch AJ, Perez-Brayfield M, Smith EA. The modified sting procedure to correct vesicoureteral reflux: improved results with submucosal implantation within the intramural ureter. J Urol 2004; 171 (6 Pt 1): 2413–2416. DOI: 10.1097/01.ju.0000127754.79866.7f.
- McMann LP, Scherz HC, Kirsch AJ. Long-term preservation of dextranomer/hyaluronic acid copolymer implants after endoscopic treatment of vesicoureteral reflux in children: a sonographic volumetric analysis. J Urol 2007; 177 (1): 320. DOI: 10.1016/j.juro.2006.08.144.
- Baydilli N, Selvi I, Pinarbasi AS. Additional VCUG-related parameters for predicting the success of endoscopic injection in children with primary vesicoureteral reflux. J Pediatr Urol 2021; 17 (1). DOI: 10.1016/j.jpurol.2020.11.018.
- Kirsch AJ, Cooper CS, Läckgren G. Non-Animal Stabilized Hyaluronic Acid/Dextranomer Gel (NASHA/Dx, Deflux) for Endoscopic Treatment of Vesicoureteral Reflux: What Have We Learned Over the Last 20 Years? Urology 2021; 157: 15–28. DOI: 10.1016/j.urology.2021.07.032.
- Routh JC, Inman BA, Reinberg Y. Dextranomer/hyaluronic acid for pediatric vesicoureteral reflux: systematic review. Pediatrics 2010; 125 (5): 10 1542 2009–2225. DOI: 10.1016/j.yped.2011.04.057.
- Elder JS, Diaz M, Caldamone AA. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. J Urol 2006; 175 (2): 10 1016 0022–5347 05 00210–00217. DOI: 10.1016/s0084-4071(08)70406-8.
- Arlen AM, Scherz HC, Filimon E. Is routine voiding cystourethrogram necessary following double hit for primary vesicoureteral reflux? J Pediatr Urol 2015; 11 (1). DOI: 10.1016/j.jpurol.2014.11.011.
- Wang PZT, Abdelhalim A, Walia A. Avoiding routine postoperative voiding cystourethrogram: Predicting radiologic success for endoscopically treated vesicoureteral reflux. Can Urol Assoc J 2019; 13 (5). DOI: 10.5489/cuaj.5589.
- Knudson MJ, Cooper CS, Block CA. Calcification of glutaraldehyde cross-linked collagen in bladder neck injections in children with incontinence: a long-term complication. J Urol 2006; 176 (3): 1143–1146. DOI: 10.1016/j.juro.2006.04.059.
- Gargollo PC, Paltiel HJ, Rosoklija I. Mound calcification after endoscopic treatment of vesicoureteral reflux with autologous chondrocytes\–a normal variant of mound appearance? J Urol 2009; 181 (6): 2707–2708. DOI: 10.1016/j.juro.2009.02.053.
- Noe HN. Calcification in a Deflux bleb thought to be a ureteral calculus in a child. J Pediatr Urol 2008; 4 (1): 88–89. DOI: 10.1016/j.jpurol.2007.02.005.
- Vandersteen DR, Routh JC, Kirsch AJ. Postoperative ureteral obstruction after subureteral injection of dextranomer/hyaluronic Acid copolymer. J Urol 2006; 176(4. DOI: 10.1016/j.juro.2006.06.101.
- Papagiannopoulos D, Rosoklija I, Cheng E. Delayed Obstruction With Asymptomatic Loss of Renal Function After Dextranomer/Hyaluronic Acid Copolymer (Deflux) Injection for Vesicoureteral Reflux: A Close Look at a Disturbing Outcome. Urology 2017; 101: 63–66. DOI: 10.1016/j.urology.2016.09.013.
- Romain J, Fourcade L, Centi J. Delayed-onset Ureteral Obstruction and Calcification Masquerading as Renal Colic Following Deflux Injection. Urology 2016; 94: 218–220. DOI: 10.1016/j.urology.2016.03.001.
- Christen S, Mendoza M, Gobet R. Late ureteral obstruction after injection of dextranomer/hyaluronic acid copolymer. Urology 2014; 83 (4): 920–922. DOI: 10.1016/j.urology.2013.10.053.
- Austin JC, Cooper CS. Vesicoureteral reflux: surgical approaches. Urol Clin North Am 2004; 31 (3): 543–557. DOI: 10.1016/j.ucl.2004.04.018.
- Sprunger JK, Reese CT, Decter RM. Can standard open pediatric urological procedures be performed on an outpatient basis? J Urol 2001; 166 (3): 1062–1064. DOI: 10.1097/00005392-200109000-00083.
- Marotte JB, Smith DP. Extravesical ureteral reimplantations for the correction of primary reflux can be done as outpatient procedures. J Urol 2001; 165 (6 Pt 2): 10 1097 00005392–200106001–00003. DOI: 10.1016/s0022-5347(05)66171-x.
- Palmer JS. Bilateral extravesical ureteral reimplantation in toilet-trained children: short-stay procedure without urinary retention. Urology 2009; 73 (2): 285–288. DOI: 10.1016/j.urology.2008.07.046.
- Barrieras D, Lapointe S, Reddy PP. Are postoperative studies justified after extravescial ureteral reimplantation? J Urol 2000; 164(3. DOI: 10.1097/00005392-200009020-00035.
- Bisignani G, Decter RM. Voiding cystourethrography after uncomplicated ureteral reimplantation in children: is it necessary? J Urol 1997; 158(3. DOI: 10.1016/s0022-5347(01)64437-9.
- El-Ghoneimi A, Odet E, Lamer S. Cystography after the Cohen ureterovesical reimplantation: is it necessary at a training center? J Urol 1999; 162(3. DOI: 10.1016/s0022-5347(01)68133-3.
- Harel M, Herbst KW, Silvis R. Objective pain assessment after ureteral reimplantation: comparison of open versus robotic approach. J Pediatr Urol 2015; 11 (2). DOI: 10.1016/j.jpurol.2014.12.007.
- Boysen WR, Ellison JS, Kim C. Multi-Institutional Review of Outcomes and Complications of Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation for Treatment of Primary Vesicoureteral Reflux in Children. J Urol 2017; 197 (6): 1555–1561. DOI: 10.1016/j.juro.2017.01.062.
- Kurtz MP, Leow JJ, Varda BK. Robotic versus open pediatric ureteral reimplantation: Costs and complications from a nationwide sample. J Pediatr Urol 2016; 12 (6). DOI: 10.1016/j.jpurol.2016.06.016.
- Bowen DK, Faasse MA, Liu DB. Use of Pediatric Open, Laparoscopic and Robot-Assisted Laparoscopic Ureteral Reimplantation in the United States: 2000 to 2012. J Urol 2016; 196 (1): 207–212. DOI: 10.1016/j.juro.2016.02.065.
- Rolleston GL, Maling TM, Hodson CJ. Intrarenal reflux and the scarred kidney. Arch Dis Child 1974; 49 (7): 531–539. DOI: 10.1136/adc.49.7.531.
- Austin JC. Treatment of vesicoureteral reflux after puberty. Adv Urol 2008; 2008 (590185). DOI: 10.1155/2008/590185.
最近更新时间: 2023-02-22 15:40